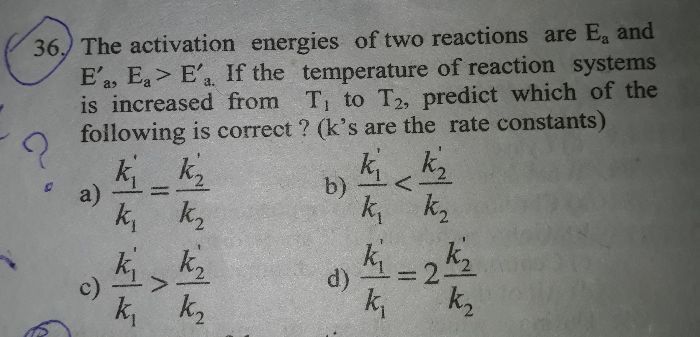CBSE Class 12-science Answered
4.80 ques
Asked by lovemaan5500 | 30 Jan, 2020, 15:29: PM
This reaction takes place in the following steps-
(i) 2NO+H2 .........> N2 + H2O2 (slow)
(ii) H2O2 + H2 .........> 2H2O (fast)
Step(i) is slow wso it rate determining step.
The rate is given by =
-dx/dt=d[N2]/dt=k[NO]2 [H2]
Answered by Ravi | 31 Jan, 2020, 13:10: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by desaianant541 | 15 May, 2024, 21:05: PM
CBSE 12-science - Chemistry
Asked by bhadauriyax | 30 Nov, 2023, 18:23: PM
CBSE 12-science - Chemistry
Asked by rahulbiswal946 | 08 Nov, 2023, 19:01: PM
CBSE 12-science - Chemistry
Asked by arshbhatia0809 | 22 Jul, 2021, 21:47: PM
CBSE 12-science - Chemistry
Asked by Surendersingh0493 | 18 Oct, 2020, 14:05: PM
CBSE 12-science - Chemistry
Asked by khandarev3580 | 10 Oct, 2020, 10:54: AM
CBSE 12-science - Chemistry
Asked by dr.akanksha0411 | 07 Aug, 2020, 11:56: AM
CBSE 12-science - Chemistry
Asked by amritha2960 | 13 May, 2020, 08:26: AM
CBSE 12-science - Chemistry
Asked by sulaikhasulu393 | 02 May, 2020, 09:20: AM
CBSE 12-science - Chemistry
Asked by leelakrishnapallapotu143 | 29 Mar, 2020, 19:27: PM