Integrated Rate Equation Free Doubts and Solutions
CBSE - XII Science - Chemistry - Chemical Kinetics
Please ans both part

CBSE - XII Science - Chemistry - Chemical Kinetics
The experimental data for the reaction: 2A + B2 → 2AB is: Write the rate equation for the reaction?
CBSE - XII Science - Chemistry - Chemical Kinetics
(a) A reaction is second order in A and first order in B. (i)Write the differential rate equation. (ii)How is the rate affected on increasing the concentration of A three times? (iii) How is the rate affected when the concentrations of both A and B are doubled? (b)A first order reaction takes 40 minutes for 30% decomposition. Calculate t1/2 for this reaction. (Given log 1.428 = 0.1548) OR (a)For a first order reaction, show that time required for 99% completion is twice the time required for the completion of 90% of reaction. (b)Rate constant 'k' of a reaction varies with temperature 'T' according to the equation: Where Ea is the activation energy. When a graph is plotted for log k Vs straight line with a slope of -4250 K is obtained. Calculate 'Ea' for the reaction. (R = 8.314 JK-1 mol-1)
CBSE - XII Science - Chemistry - Chemical Kinetics
The rate constant for a reaction of zero order in A is 0.0030 mol L-1 s-1. How long will it take for the initial concentration of A to fall from 0.10 M to 0.075 M?
CBSE - XII Science - Chemistry - Chemical Kinetics
Derive an equation for calculating the half life of a first order reaction
CBSE - XII Science - Chemistry - Chemical Kinetics
Calculate the rate of reaction from the rate law: = k[A] [B]2, when the concentration of A and B are 0.01 M and 0.02 M respectively and k = 5.1 x 10-3 L2 mol-2 s-1.
CBSE - XII Science - Chemistry - Chemical Kinetics
In second order reaction why the graph btwn concentration and rate will be parabola and why it will be starting from origin and will open upwards?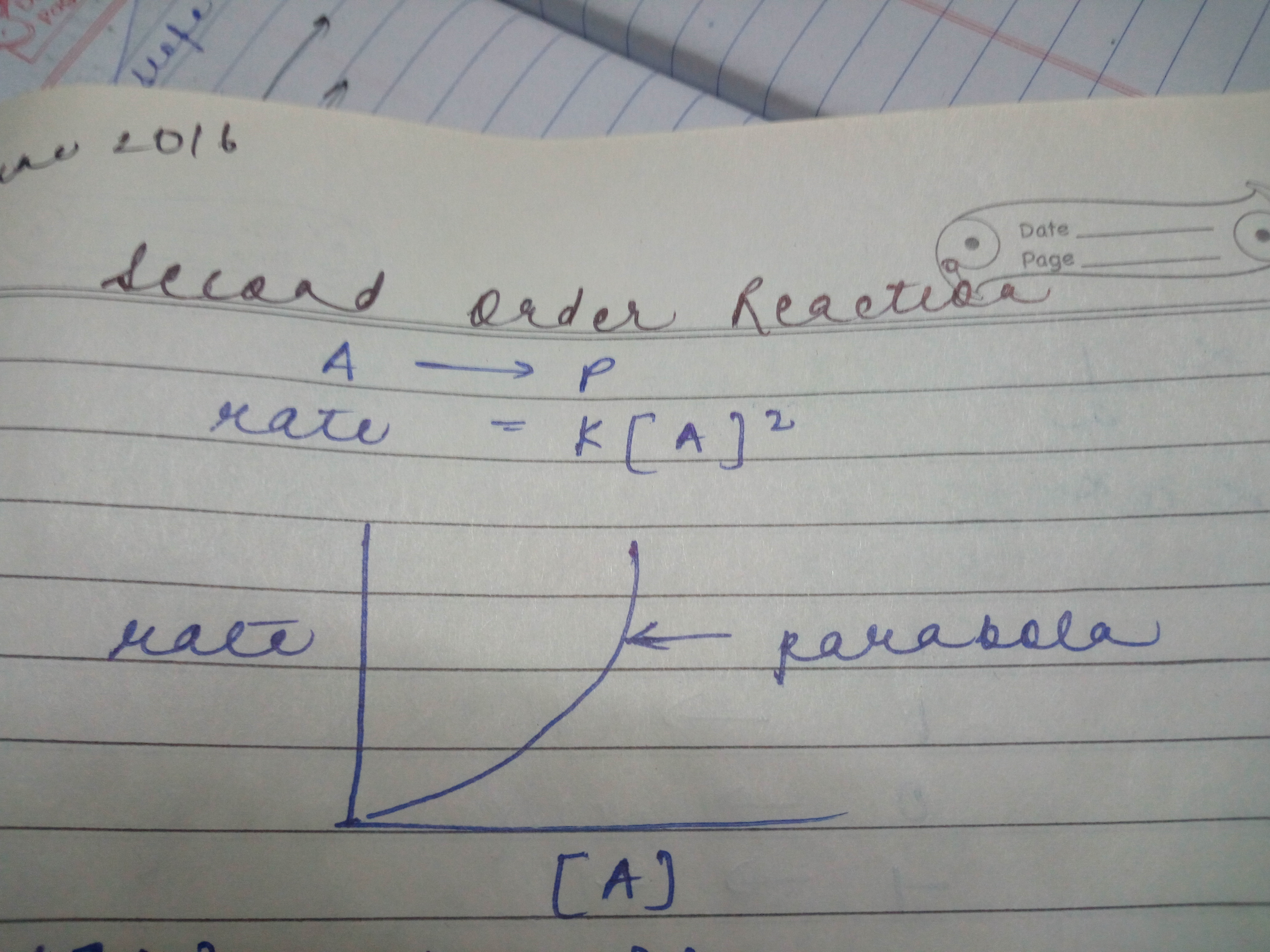

CBSE - XII Science - Chemistry - Chemical Kinetics
IN A RADIOACTIVE EQUILIBRIUM 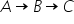 , THE RATIO OF NUMBER OF ATOMS OF A TO B IS
, THE RATIO OF NUMBER OF ATOMS OF A TO B IS 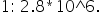
IF HALF LIFE OF A IS 1620 YEARS, WHAT IS THE HALF LIFE OF B?
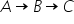 , THE RATIO OF NUMBER OF ATOMS OF A TO B IS
, THE RATIO OF NUMBER OF ATOMS OF A TO B IS 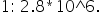
CBSE - XII Science - Chemistry - Chemical Kinetics
Half life of reaction : H2O2(aq)= H2O(l) + 1/2 O2 (g) is independent of initial concentration of H2O2 volume of O2 gas after 20 minute is 5L at 1 atm and 27degreeC and after completion of reaction is 50L. The rate constant is :
(1) 1/20 log 10 min ^-1
(2) 2.303/20 log 10 min ^-1
(3) 2.303/20 log 50/45 min ^-1
(4) 2.303/20 log 45/50 min ^-1
CBSE - XII Science - Chemistry - Chemical Kinetics
An optically active compound 'A' upon acid catalysed hydrolysis yield two optically active compounds 'B' and 'C' by pseudo first order kinetics. The observed rotation of the mixture after 20 minutes was 5 degrees and after completion of reaction was -20 degrees. If optical rotation per mole of A, B and C are 60, 40 and -80 degrees. Calculate half life of the reaction.
CBSE - XII Science - Chemistry - Chemical Kinetics
how to find log
CBSE - XII Science - Chemistry - Chemical Kinetics
The decomposition of Cl2O7 at 400K in gas phase to Cl2 and O2 is a first order reaction.
(i) After 55s at 400K, the pressure of Cl2O7 falls from 0.062 to 0.044 atm. Calculate the
rate constant.
(ii) Calculate the pressure of Cl2O7 after 100s of decomposition at this temperature.
(i) After 55s at 400K, the pressure of Cl2O7 falls from 0.062 to 0.044 atm. Calculate the
rate constant.
(ii) Calculate the pressure of Cl2O7 after 100s of decomposition at this temperature.

