CBSE Class 12-science Answered
In second order reaction why the graph btwn concentration and rate will be parabola and why it will be starting from origin and will open upwards?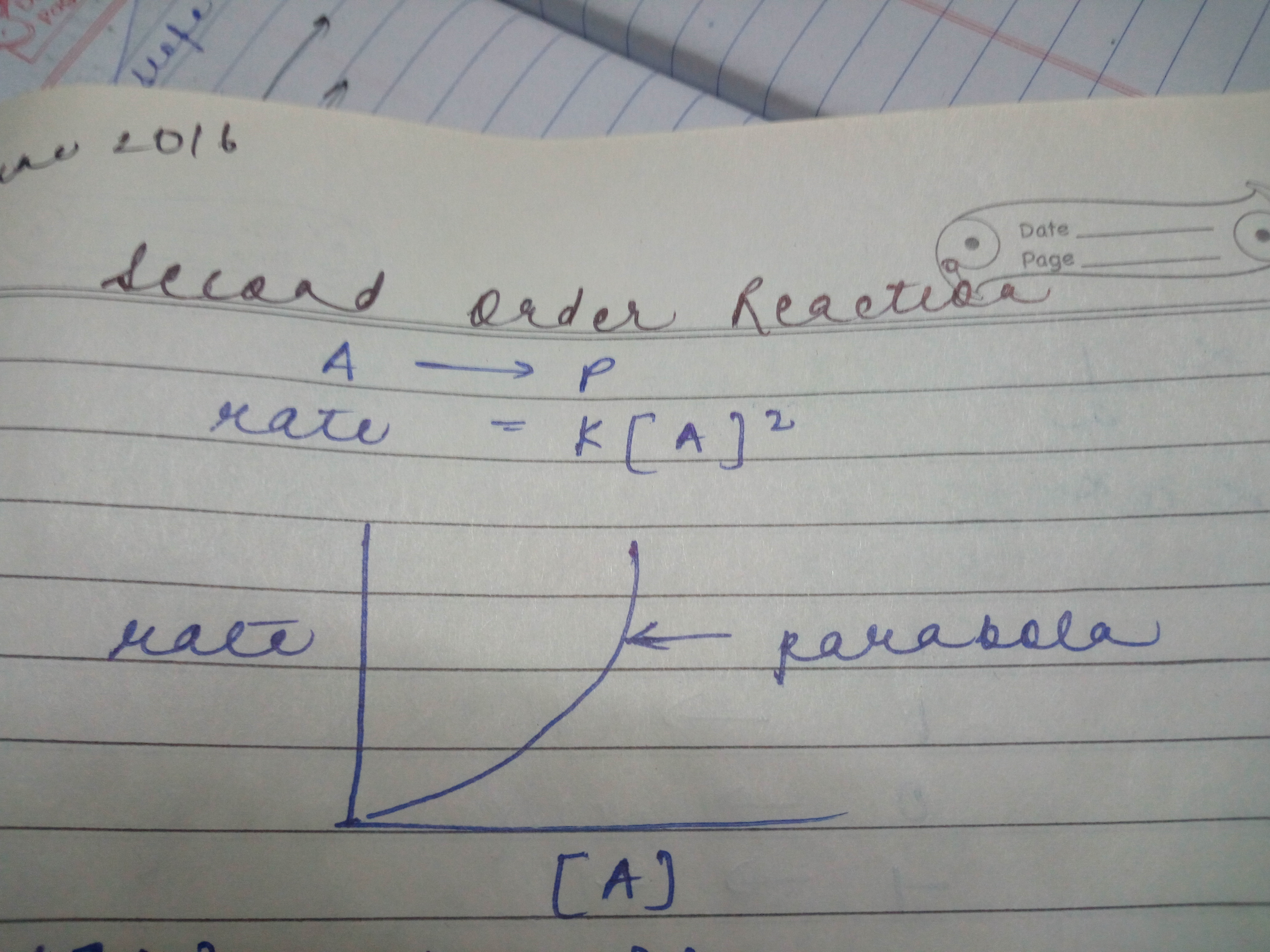

Asked by ayushi | 12 Jun, 2016, 09:03: PM
Dear ayushicutiepie21@gmail.com
Thanks for asking us a question in Ask the Expert section of TopperLearning.com.
If the rate is proportional to some power of the concentration greater than one you will get parabola. In case of second order reaction rate is propotional to [A]2 . At very, very low concentrations, the rate is proportional to the substrate concentration. But as concentration increases, increasing the concentration more has less and less effect - and eventually the rate reaches a maximum.
Regards
Topperlearning Team.
Topperlearning Team.
Answered by Arvind Diwale | 13 Jun, 2016, 12:37: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by amitkumar.cis | 14 Jan, 2021, 12:40: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 28 Mar, 2014, 10:16: AM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 22 Jun, 2016, 09:20: AM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 22 Jun, 2016, 09:17: AM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 22 Jun, 2016, 09:18: AM





