Order And Molecularity Of Reaction Free Doubts and Solutions
CBSE - XII Science - Chemistry - Chemical Kinetics
relation between k and t for first order reaction

CBSE - XII Science - Chemistry - Chemical Kinetics
Definition of zero order, 1st order and 2nd order reaction
CBSE - XII Science - Chemistry - Chemical Kinetics
The half life for a first order reaction is 10 mins .What percentage of reactant will be left behind after 60mins.
CBSE - XII Science - Chemistry - Chemical Kinetics
What zero order reaction
CBSE - XII Science - Chemistry - Chemical Kinetics
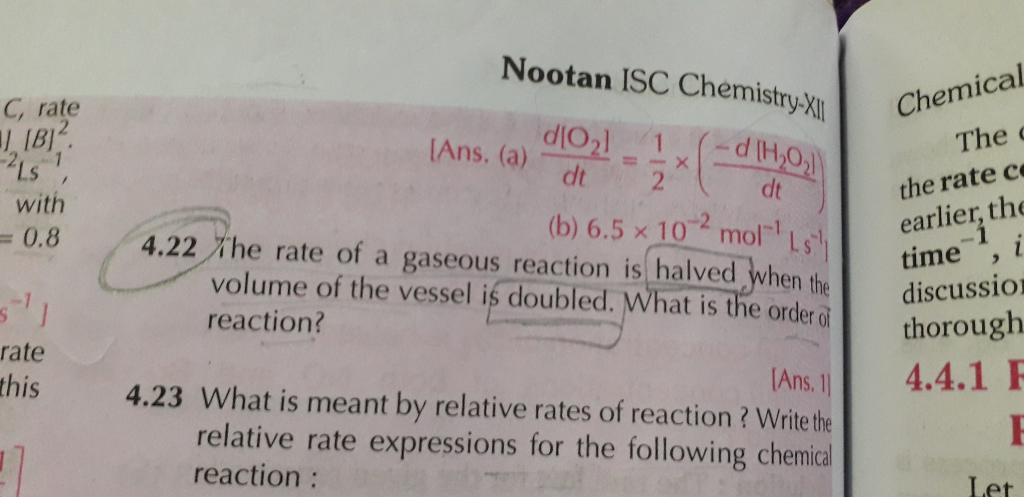
4.22 sum
CBSE - XII Science - Chemistry - Chemical Kinetics
4.32 numerical plz

CBSE - XII Science - Chemistry - Chemical Kinetics
Please answer

CBSE - XII Science - Chemistry - Chemical Kinetics
4.32 numerical plz

CBSE - XII Science - Chemistry - Chemical Kinetics
Write a condition under which a bimolecular reaction is kinetically first order. Give an example of such a reaction
CBSE - XII Science - Chemistry - Chemical Kinetics
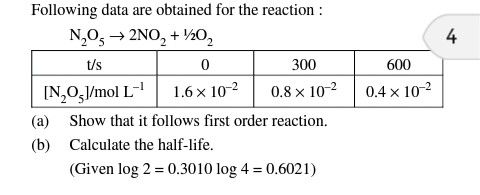
Pls answer
CBSE - XII Science - Chemistry - Chemical Kinetics
what is the order of reaction if rate of reaction becomes 3 times and concentration of reaction becomes 9 times
CBSE - XII Science - Chemistry - Chemical Kinetics
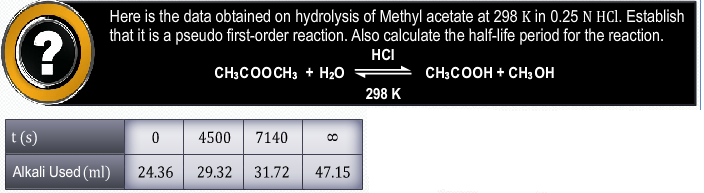
please solve it
CBSE - XII Science - Chemistry - Chemical Kinetics
For which type of reactions order and molecularity have the same value?
CBSE - XII Science - Chemistry - Chemical Kinetics
how to identify the orders of any reaction?
CBSE - XII Science - Chemistry - Chemical Kinetics
how can we determine a reaction whether its a first , second , third , zero, or fraction order reaction?
CBSE - XII Science - Chemistry - Chemical Kinetics
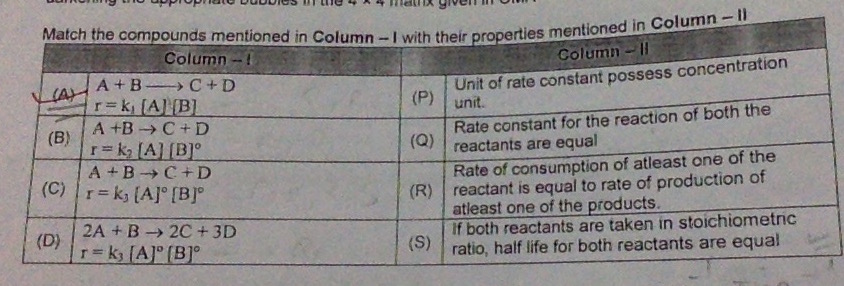
Q) what will the correct match of (B) & also explain the reason (one or more than one correct )