Chemical Kinetics Free Doubts and Solutions
CBSE - XII Science - Chemistry - Chemical Kinetics
kp and kc
CBSE - XII Science - Chemistry - Chemical Kinetics
If a reaction with t1 = 69.3 second ; has a ra constant 10-2 per socond the order is
CBSE - XII Science - Chemistry - Chemical Kinetics
Need help with this Chemical Kinetics problem. How do I work out the decomposition rate of [N2O5]?
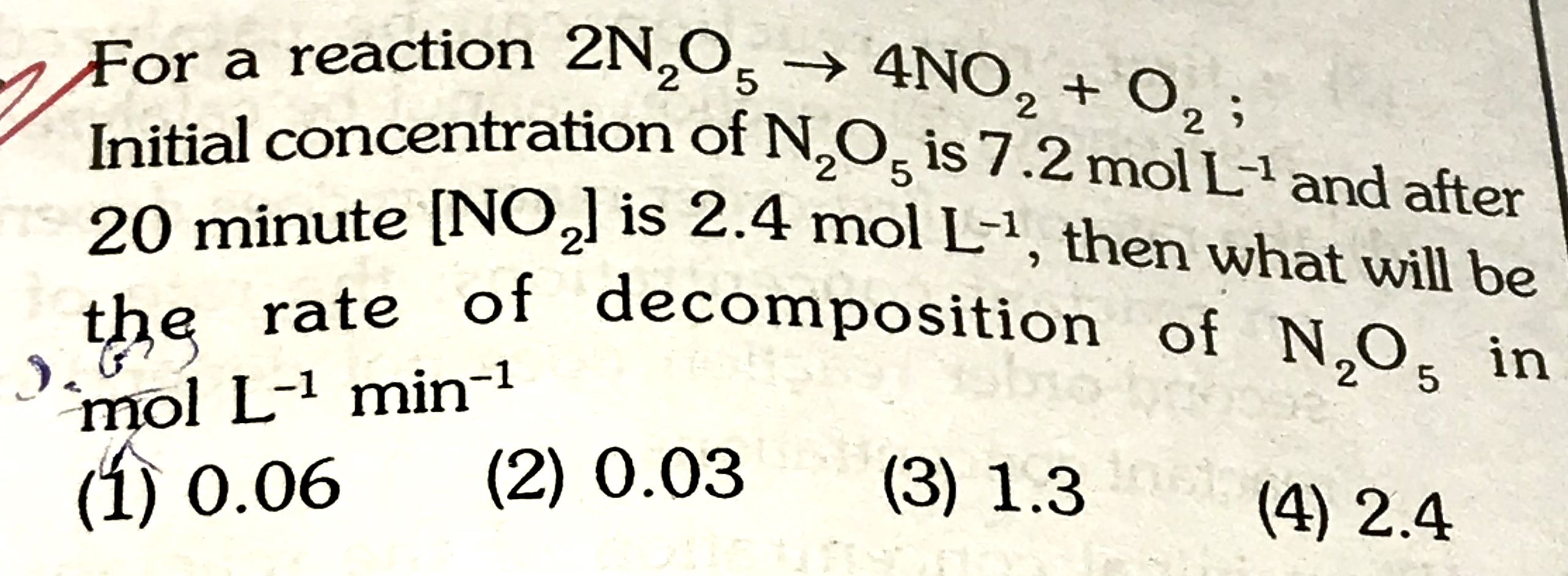
CBSE - XII Science - Chemistry - Chemical Kinetics
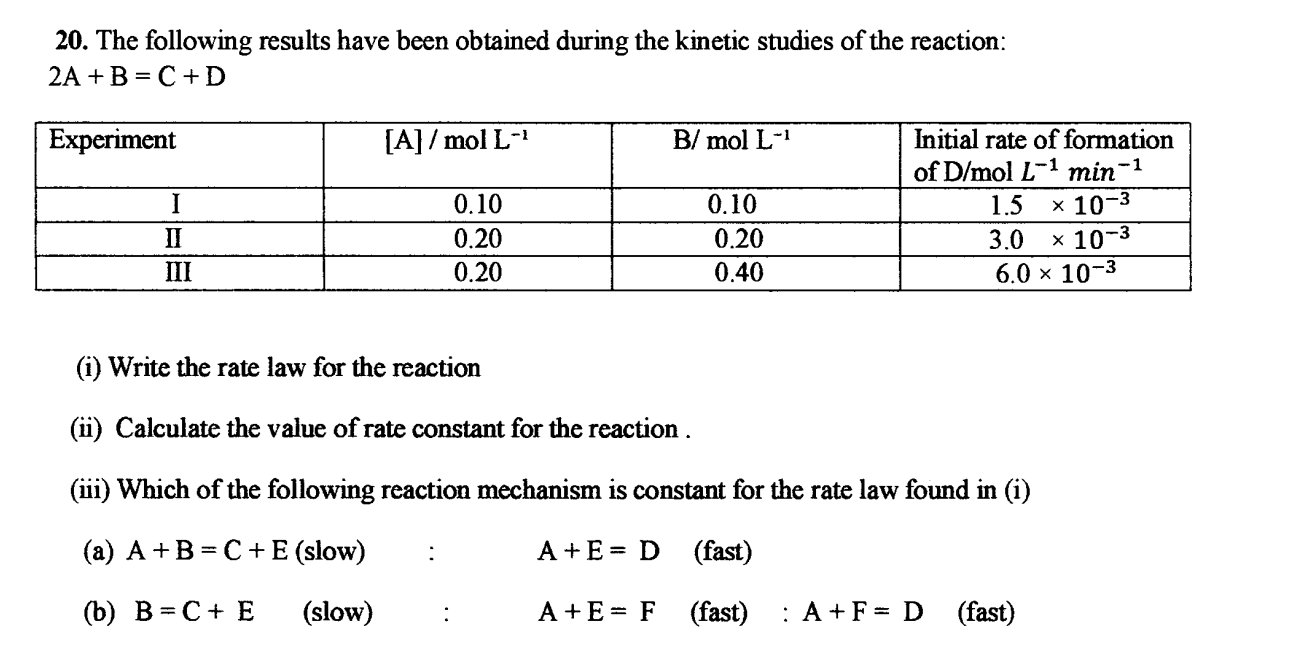 how to solve all this?
how to solve all this?
 how to solve all this?
how to solve all this?CBSE - XII Science - Chemistry - Chemical Kinetics
the rate constant for first order decomposition of H2O2 os given by the following equation
Log k=14.34-1.25*10 to the power 4 k/T calculate Ea for given reaction and at what temperature will its half period be 256 minutes?
please solve it by arrhineus equation
CBSE - XII Science - Chemistry - Chemical Kinetics
HOW TO SOLVE THIS EQUATION
T=2.303/60*LOG16/1
IT IS OF FORMULA T=2.303/K*LOG[R]@/[R]
CBSE - XII Science - Chemistry - Chemical Kinetics
what is hypothetical reaction?
CBSE - XII Science - Chemistry - Chemical Kinetics
what is hypothetical reaction?
CBSE - XII Science - Chemistry - Chemical Kinetics
sir,
while calculating integrated rate equation of first order reaction, when we integrate 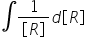 we get
we get 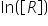 but in maths we studied that it equates to Log [R] why is it so????????????? and what is the actual relation between In and Log???????????
but in maths we studied that it equates to Log [R] why is it so????????????? and what is the actual relation between In and Log???????????
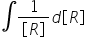 we get
we get 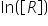 but in maths we studied that it equates to Log [R] why is it so????????????? and what is the actual relation between In and Log???????????
but in maths we studied that it equates to Log [R] why is it so????????????? and what is the actual relation between In and Log???????????CBSE - XII Science - Chemistry - Chemical Kinetics
Sir,
can you please tell me that how to find the order of reactants while calculating order of reaction. Suppose a reaction
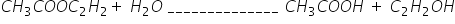 in this reaction how do we know the order of reactants????????????????
in this reaction how do we know the order of reactants????????????????
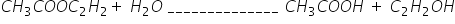 in this reaction how do we know the order of reactants????????????????
in this reaction how do we know the order of reactants???????????????? 
