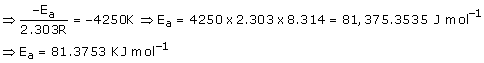CBSE Class 12-science Answered
(a)(i)
Differential rate equation: Rate =![]()
(ii) On increasing the concentration of A three times as 3A:
Rate'=![]()
I.e. New rate is 9 times the initial rate.
(iii) On increasing the concentration of A and B as 2A and 2B:
Rate"=![]()
8 times the initial rate.
(b)
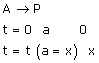
Now, it takes 40 min for 30% decomposition i.e. reactant left after 40 min is 70% of its initial concentration.
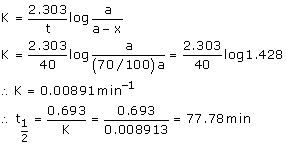
Or

Case 1: If 't' is the time required for 99% completion then x = 99% of a
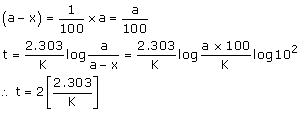
Case 2: If 't' is the time required for 90% of completion then x = 90% of a
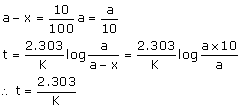
Therefore, the time required for 99% completion of 1st order reaction is twice the time required for 90% completion.
(b)![]()
Ea is Activation energy
The above equation is like y = mx + c where if we plot y v/s x we get a straight line with slope 'm' and intercept 'c'.
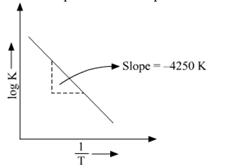
So, slope is equal to = ![]()