CBSE Class 12-science Answered
Calculate the rate of reaction from the rate law: 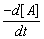 = k[A] [B]2, when the concentration of A and B are 0.01 M and 0.02 M respectively and k = 5.1 x 10-3 L2 mol-2 s-1.
= k[A] [B]2, when the concentration of A and B are 0.01 M and 0.02 M respectively and k = 5.1 x 10-3 L2 mol-2 s-1.
Asked by Topperlearning User | 22 Jun, 2016, 09:17: AM
Rate of reaction,
= 2.04 x 10-8 Ms-1
Answered by | 22 Jun, 2016, 11:17: AM
Concept Videos
CBSE 12-science - Chemistry
Asked by amitkumar.cis | 14 Jan, 2021, 12:40: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 28 Mar, 2014, 10:16: AM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 22 Jun, 2016, 09:20: AM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 22 Jun, 2016, 09:17: AM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 22 Jun, 2016, 09:18: AM





