CBSE Class 12-science Answered
The half-life of a first order decomposition of nitramide is 2.1 hour at 25o C. Determine the time taken for the compound to decompose 99% of its original amount, rate constant = 0.2303 per hour.
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
Here a = 100
a – x = 100 – 99 = 1
t = 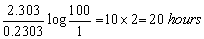
Answered by | 04 Jun, 2014, 03:23: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by amitkumar.cis | 14 Jan, 2021, 12:40: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 28 Mar, 2014, 10:16: AM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 22 Jun, 2016, 09:20: AM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 22 Jun, 2016, 09:17: AM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 22 Jun, 2016, 09:18: AM





