CBSE Class 12-science Answered
The rate of reaction between A and B increases by a factor of 100. Calculate the order of the reaction when the concentration of A is increased 10 times.
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
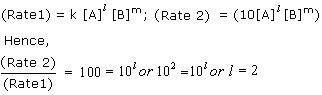
Answered by | 04 Jun, 2014, 03:23: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by amitkumar.cis | 14 Jan, 2021, 12:40: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 28 Mar, 2014, 10:16: AM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 22 Jun, 2016, 09:20: AM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 22 Jun, 2016, 09:17: AM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 22 Jun, 2016, 09:18: AM





