CBSE Class 12-science Answered
Calculate the approximate number of unit cells Present in 1gm of Gold. Gold crystallizes in FCC @ AT mass of gold = 197u. ?
Asked by vikmandal000 | 11 Feb, 2016, 03:40: PM
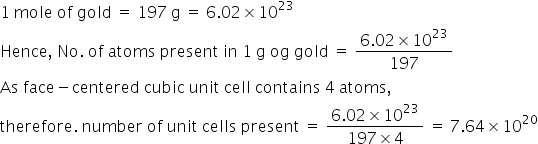
Answered by Prachi Sawant | 12 Feb, 2016, 02:43: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by ap996969 | 24 Jan, 2019, 07:08: PM
CBSE 12-science - Chemistry
Asked by shivamsaraswat484 | 23 Mar, 2018, 09:37: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 01 Apr, 2014, 01:38: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 17 Jun, 2016, 10:00: AM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 17 Jun, 2016, 10:11: AM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM




