CBSE Class 12-science Answered
give a relationship between nearest neighbour distance(d),radius of atom(r), edge of unit cell(a), for fcc and BCC crystal
Asked by ap996969 | 24 Jan, 2019, 19:08: PM
In the simple cubic lattice, the nearest neighbours touch along the edge.
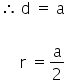
In the body centred cubic lattice (bcc) the nearest neighbours touch along the body diagonal.
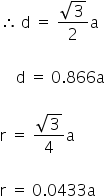
In the face centred cubic lattice (fcc) the nearest neighbours touch along the face diagonal.
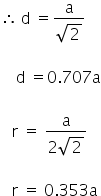
Answered by Varsha | 25 Jan, 2019, 11:43: AM
Concept Videos
CBSE 12-science - Chemistry
Asked by ap996969 | 24 Jan, 2019, 19:08: PM
CBSE 12-science - Chemistry
Asked by shivamsaraswat484 | 23 Mar, 2018, 21:37: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 01 Apr, 2014, 13:38: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 17 Jun, 2016, 10:00: AM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 17 Jun, 2016, 10:11: AM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM




