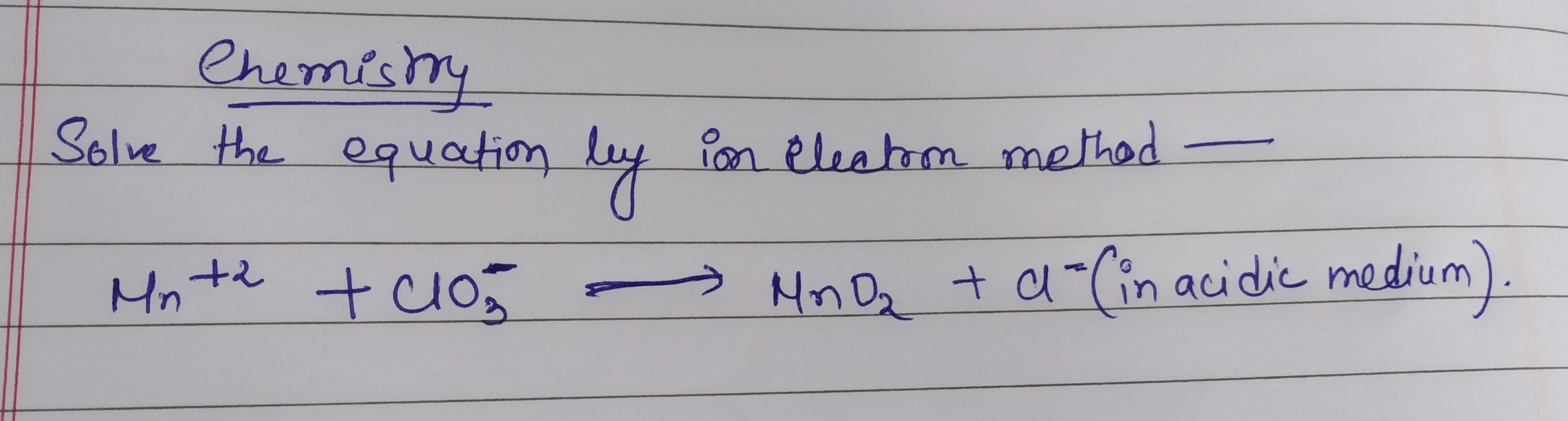
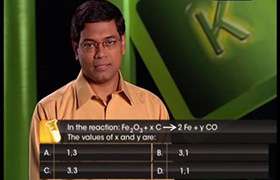CBSE Class 11-science Answered
balance the equation
1-nitrate ions in acidic medium oxidise mg to mg2+ ions but itself get reduced to nitrogenI oxide.
2-zinc reacts with conc.nitric acid to produce zinc nitrate nitrogen dioxide and water.
balance by ion electron method
3-permanganate ion oxidises oxalate ions in acidic medium to carbon dioxide and get reduced itself to mn2+
4-zinc reacts with nitric acid to form zinc II ions ,ammonium ions and water in acidic medium.
5-bromine and hydrogen peroxide react to give bromate ions and water in acidic medium.
Asked by archana | 26 Sep, 2015, 05:13: PM
Dear archusudha2013@gmail.com
Thanks for asking us a question in Ask the Expert section of TopperLearning.com.
In case of multiple questions within a query, please post each question individually and let us know where you are getting stuck so that we would be able to explain things better.
Solution,
Zinc reacts with conc. nitric acid to produce zinc nitrate, nitrogen dioxide and water.

Regards
Topperlearning Team.
Topperlearning Team.
Answered by Prachi Sawant | 26 Sep, 2015, 11:26: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by indranibaudya | 03 Oct, 2020, 08:12: PM
CBSE 11-science - Chemistry
Asked by Punshibakhuraijam2015 | 22 Sep, 2019, 08:40: PM
CBSE 11-science - Chemistry
Asked by kkdmmdsd | 09 Jun, 2019, 04:27: PM
CBSE 11-science - Chemistry
Asked by dheerajmathpal374 | 15 Sep, 2018, 10:43: AM
CBSE 11-science - Chemistry
Asked by govtsecschoolnayaganv051 | 19 Aug, 2018, 06:29: PM
CBSE 11-science - Chemistry
Asked by govtsecschoolnayaganv051 | 18 Aug, 2018, 05:08: PM
CBSE 11-science - Chemistry
Asked by govtsecschoolnayaganv051 | 14 Aug, 2018, 06:06: PM
CBSE 11-science - Chemistry
Asked by vaagai2353 | 29 Jun, 2018, 10:29: PM
CBSE 11-science - Chemistry
Asked by g_archanasharma | 22 Mar, 2018, 10:11: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM