CBSE Class 11-science Answered
An electron in the hydrogen atom jumps from excited state n to the ground state.The wavelength so emitted illuminates a photo-sensitive material having work function 2.75eV.If the stopping potential of the photoelectron is 10V, the value of n is
Asked by aiswaryapbindhu3 | 08 Aug, 2018, 11:40: PM
Einstein's photo-electric equation is given by, eVo = hν - φ0 ............(1)
where e is electronic charge, Vo is stopping potential, h is plancks constant, ν is frequency of radiation and φ0 is work function .
if we consider hν as the difference between energy En of nth excited state and ground state(n=1), we rewrite eqn.(1) as
En = eVo + φo = 10+2.75 = 12.75 eV ...................(2)
Energy difference En between nth excited state and ground state is given by, 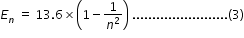
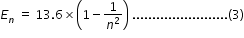
from (2) and (3), we have 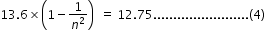
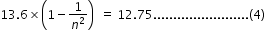
solving for n in eqn.(4), we get n = 4
Answered by Thiyagarajan K | 09 Aug, 2018, 11:41: AM
CBSE 11-science - Physics
Asked by sheikhsaadat24 | 17 Apr, 2024, 09:41: PM
CBSE 11-science - Physics
Asked by sy123946 | 07 Apr, 2024, 04:23: PM
CBSE 11-science - Physics
Asked by derhebha955 | 03 Apr, 2024, 09:03: AM
CBSE 11-science - Physics
Asked by sumedhasingh238 | 29 Mar, 2024, 05:15: PM
CBSE 11-science - Physics
Asked by sumedhasingh238 | 28 Mar, 2024, 11:10: PM
CBSE 11-science - Physics
Asked by roshnibudhrani88 | 23 Mar, 2024, 05:52: PM
CBSE 11-science - Physics
Asked by emad.amd | 21 Mar, 2024, 12:00: PM
CBSE 11-science - Physics
Asked by vinitdubey7735 | 14 Mar, 2024, 11:21: AM
CBSE 11-science - Physics
Asked by om636694 | 04 Mar, 2024, 09:10: PM
CBSE 11-science - Physics
Asked by rajuinwati12 | 04 Mar, 2024, 09:22: AM




