NEET Class neet Answered
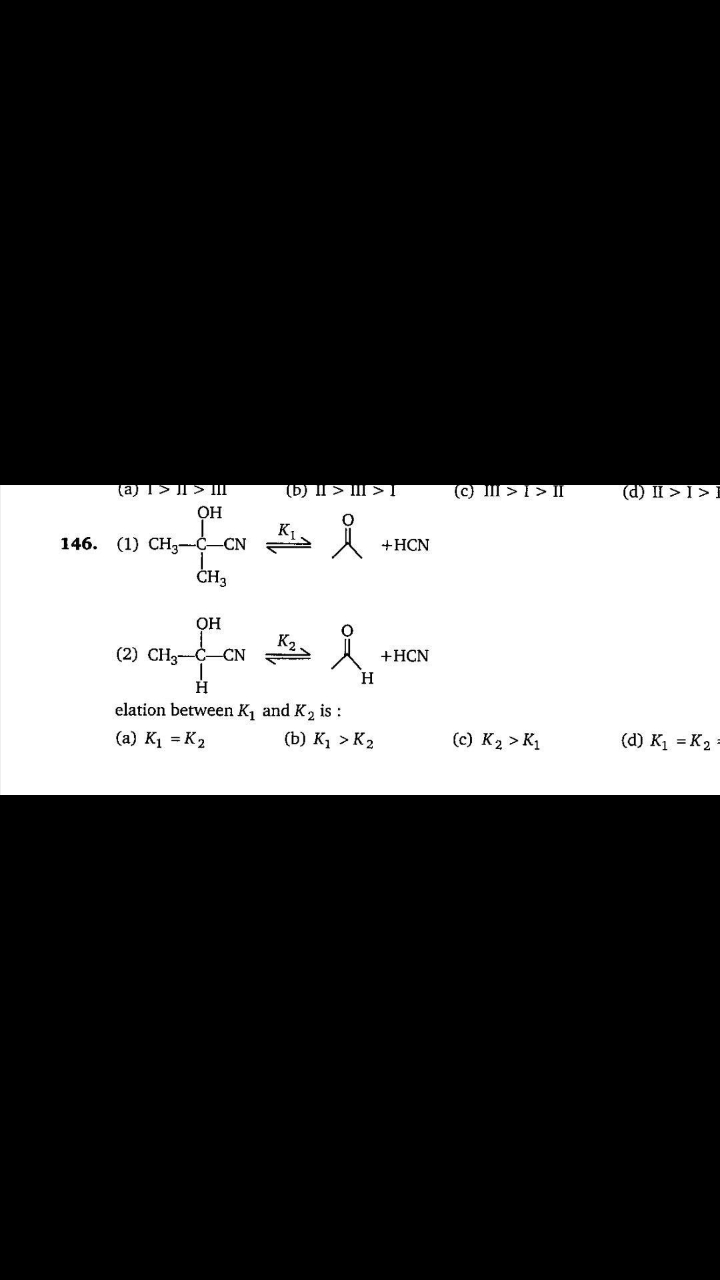
A solution is 0.1M with respect to Ag+, Ca2+, Mg2+ and Al3+ which will precipitate at lowest concentration of [PO4^3-] when solution of Na3PO4 is added? 1) Ag3Ag3PO4(Ksp=1*10^-6), 2) Ca3(PO4)2(Ksp=1*10^-33), 3) Mg3(PO4)2(Ksp=1*10^-24), 4) AlPO4(Ksp=1*10^-20)
Asked by patra04011965 | 01 Jan, 2019, 10:39: PM
Ans: option (4)
Foe AlPO4,
Ksp = [Al3+] [PO4-3 ]
Ksp = [0.1] [PO4-3 ]
[Al3+] [PO4-3 ] = 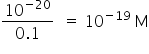
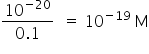
Answered by Ramandeep | 07 Jan, 2019, 01:08: PM
NEET neet - Chemistry
Asked by 8239682116rahul | 10 Apr, 2024, 01:48: PM
NEET neet - Chemistry
Asked by ramadevisupriya5678 | 28 Mar, 2024, 02:18: PM
NEET neet - Chemistry
Asked by myindiaisbad | 17 Jun, 2022, 11:17: AM
NEET neet - Chemistry
Asked by bhaveshkaria31 | 30 May, 2022, 09:26: PM
NEET neet - Chemistry
Asked by rautganesh2255 | 01 Jul, 2021, 09:32: AM
NEET neet - Chemistry
Asked by NituBarman192 | 01 Jun, 2021, 10:22: PM
NEET neet - Chemistry
Asked by bhagirathdangi12345 | 12 Feb, 2021, 01:42: PM
NEET neet - Chemistry
Asked by akashmanu09 | 08 Jan, 2021, 10:21: AM
NEET neet - Chemistry
Asked by prakriti12oct | 28 Apr, 2020, 01:31: AM