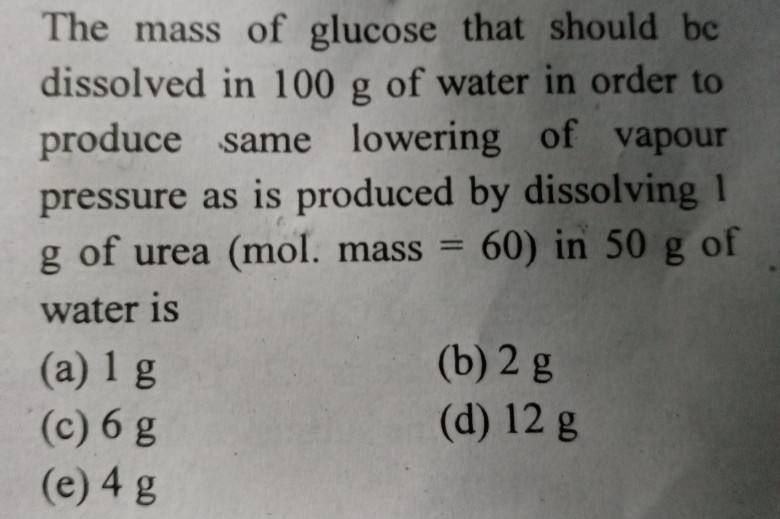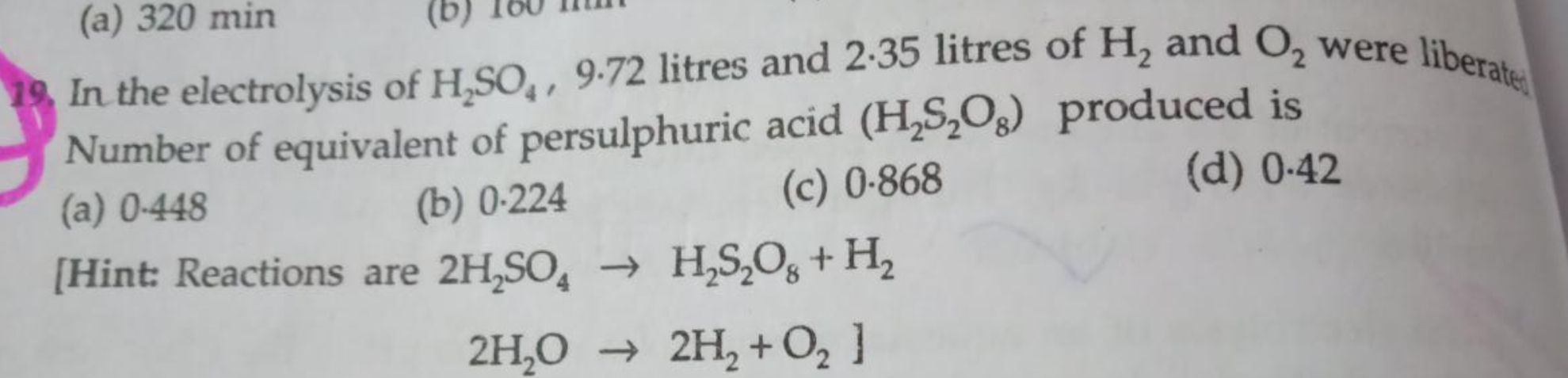NEET Class neet Answered
the boiling point of 0.15 molar aqueous solution of an unknown solute is 373.23 K at 1 atm . The molal elevatio constant of water is ______ K kg mol ^(-1)
Asked by rautganesh2255 | 01 Jul, 2021, 09:32: AM
Dear Student,
This question is based on concept of 'Elevation in Boiling Point'.
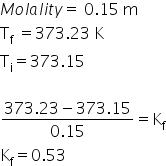
Answered by Ravi | 08 Jul, 2021, 08:36: PM
NEET neet - Chemistry
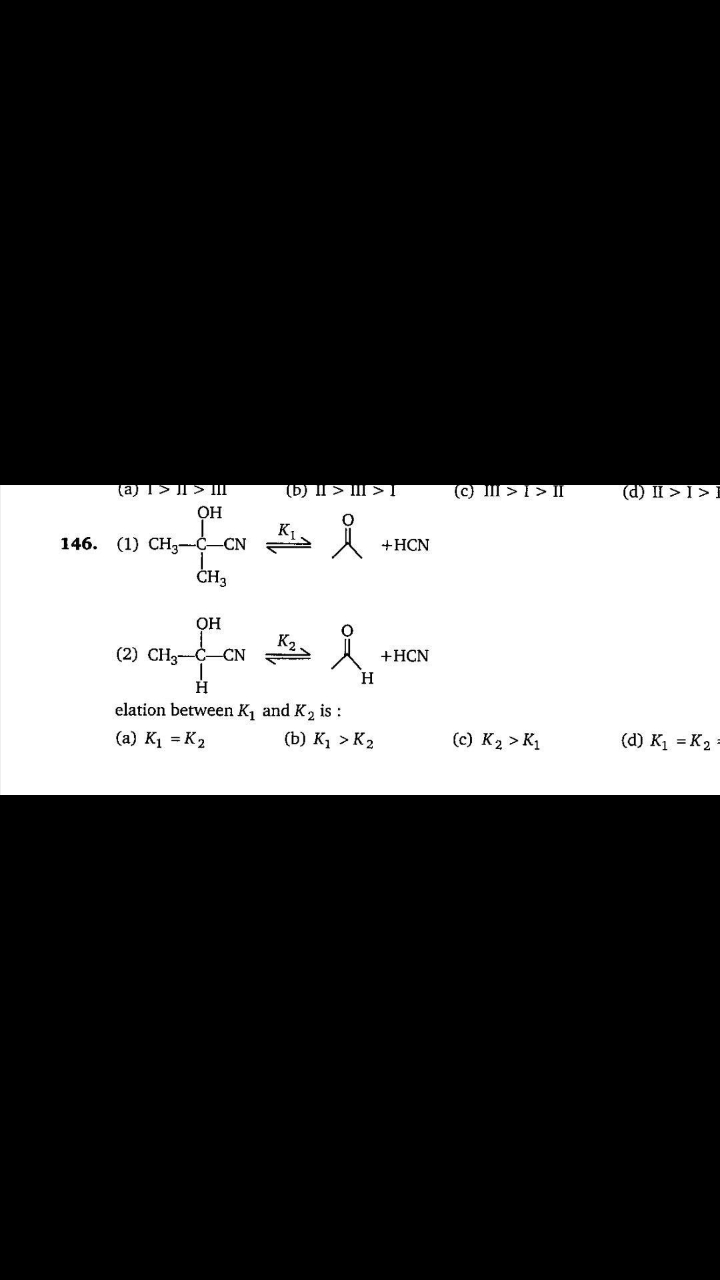
Asked by 8239682116rahul | 10 Apr, 2024, 01:48: PM
NEET neet - Chemistry
Asked by ramadevisupriya5678 | 28 Mar, 2024, 02:18: PM
NEET neet - Chemistry
Asked by myindiaisbad | 17 Jun, 2022, 11:17: AM
NEET neet - Chemistry
Asked by bhaveshkaria31 | 30 May, 2022, 09:26: PM
NEET neet - Chemistry
Asked by rautganesh2255 | 01 Jul, 2021, 09:32: AM
NEET neet - Chemistry
Asked by NituBarman192 | 01 Jun, 2021, 10:22: PM
NEET neet - Chemistry
Asked by bhagirathdangi12345 | 12 Feb, 2021, 01:42: PM
NEET neet - Chemistry
Asked by akashmanu09 | 08 Jan, 2021, 10:21: AM
NEET neet - Chemistry
Asked by prakriti12oct | 28 Apr, 2020, 01:31: AM