CBSE Class 12-science - Temperature Dependence of Reaction Videos
Rate of Reactions
This video describes the effect of temperature and use of catalyst on rate of reactions.
More videos from this chapter
View All- the half life span of a radioactive element is 10 years .in no. will 10 gram of that substances remain 1•25 gram
- what is the formula for effect of temperature on rate of reaction?
- Define activation energy.
- What is the effect of a catalyst on the equilibrium constant of a reaction?
- What is temperature coefficient? Explain
- Define transition state or activation complex.
- The activation energy for a reaction is zero. Calculate the value of its rate constant at 300 K, if k = 1.6 x 106 s-1 at 280 K? (R = 8.31 JK-1 Mol-1)
-
Draw a graph to show the various energy changes that take place in the following reaction:
H2 (g) + I2 (g)
 2HI (g)
2HI (g)
- Explain why the activation energy of a reaction can never be zero.
-
The rate constant for the first order decomposition of certain reaction is described by the equation log k (sec-1) =
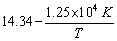 Calculate the activation energy for this reaction.
Calculate the activation energy for this reaction.






