CBSE Class 12-science Answered
what is the formula for effect of temperature on rate of reaction?
Asked by imtiyazmulla68 | 22 Mar, 2018, 08:25: PM
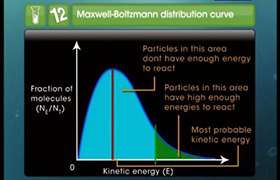
The temperature dependence of the rate of a chemical reaction can be accurately explained by Arrhenius equation,

Where,
A = Arrhenius factor or the frequency factor or pre-exponential factor.
R = Gas constant = 8.314 JK-1mol-1
Ea = Activation energy in Jmol-1
Answered by Varsha | 23 Mar, 2018, 09:44: AM
Concept Videos
CBSE 12-science - Chemistry
Asked by mishraridhi2020 | 23 Jun, 2022, 09:16: AM
CBSE 12-science - Chemistry
Asked by imtiyazmulla68 | 22 Mar, 2018, 08:25: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 22 Jun, 2016, 10:39: AM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 22 Jun, 2016, 09:56: AM