CBSE Class 12-science Answered
Explain why the activation energy of a reaction can never be zero.
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
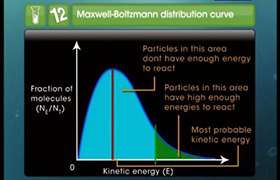
The Activation energy of a reaction can never be zero because if Ea = 0, then the expression k = A.e -Ea/RT = A.eo = A
In other words the rate constant becomes equal to collision factor. This implies that every collision results into a chemical product. This cannot be true.
In other words the rate constant becomes equal to collision factor. This implies that every collision results into a chemical product. This cannot be true.
Answered by | 04 Jun, 2014, 03:23: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by mishraridhi2020 | 23 Jun, 2022, 09:16: AM
CBSE 12-science - Chemistry
Asked by imtiyazmulla68 | 22 Mar, 2018, 08:25: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 22 Jun, 2016, 10:39: AM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 22 Jun, 2016, 09:56: AM