CBSE Class 12 science: Temperature Dependence of Reaction Videos | Temperature dependence of reaction
Temperature dependence of reaction
This video describes the effect of temperature and use of catalyst on the rate of reactions.
More videos from this chapter
View All- the half life span of a radioactive element is 10 years .in no. will 10 gram of that substances remain 1•25 gram
- what is the formula for effect of temperature on rate of reaction?
- Define activation energy.
- What is the effect of a catalyst on the equilibrium constant of a reaction?
- What is temperature coefficient? Explain
- Define transition state or activation complex.
- The activation energy for a reaction is zero. Calculate the value of its rate constant at 300 K, if k = 1.6 x 106 s-1 at 280 K? (R = 8.31 JK-1 Mol-1)
-
Draw a graph to show the various energy changes that take place in the following reaction:
H2 (g) + I2 (g)
 2HI (g)
2HI (g)
- Explain why the activation energy of a reaction can never be zero.
-
The rate constant for the first order decomposition of certain reaction is described by the equation log k (sec-1) =
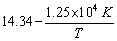 Calculate the activation energy for this reaction.
Calculate the activation energy for this reaction.





