CBSE Class 11-science Physics Law of Equipartition of Energy
- Does the average K.E per module of the gas depend upon the mass of the molecule?
- What do you mean by the number of degrees of freedom of a dynamical system? How many degrees of freedom are associated with (i) a bob of an oscillating simple pendulum (ii) an insect moving on a horizontal floor (iii) a buzzing bee (iv) a particle moving in a plane
- State the factors on which mean free path (λ) of a gas molecule depends.
- What is meant by the term ' Molar specific heat ' of a gas? The molar specific heat of hydrogen in the temperature range of about 250 K to 750 K is about (5/2) R. At lower temperatures the value of molar specific heat of hydrogen decreases to the value typical of monoatomic gases (3/2) R while at higher temperatures, it tends to the value (7/2) R. Explain.
- Find the average increase in kinetic energy of a molecule of gas per unit rise in temperature.
- (a) How many degrees of freedom are there due to vibration motion of diatomic gas molecules? (b) How many degrees of freedom are there for monoatomic, diatomic and triatomic gas due to translational and rotational motion only?
- Using the law of equipartition of energy, calculate the total energy of one mole of monoatomic, diatomic and triatomic gases?
- The average number of degree of freedom of an ideal gas molecule is six. The gas absorbs 120 J of heat at constant pressure. Find the increase in internal energy of gas.
- The average number of degree of freedom of an ideal gas molecules is five. The gas does 20J of work at constant pressure. Find the increase in internal energy and heat absorbed by gas.
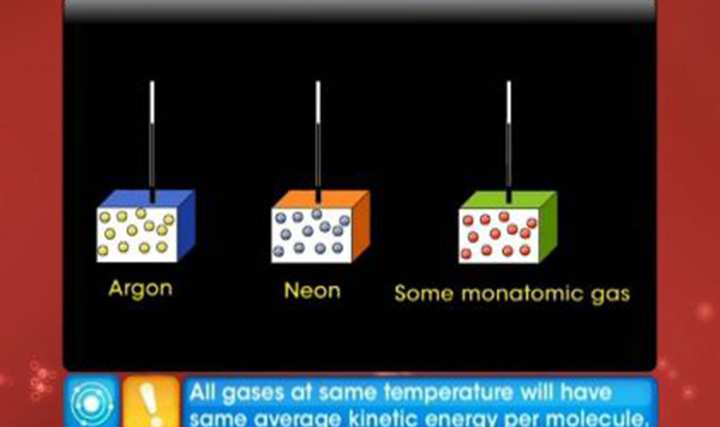
- State law of equipartition of energy.