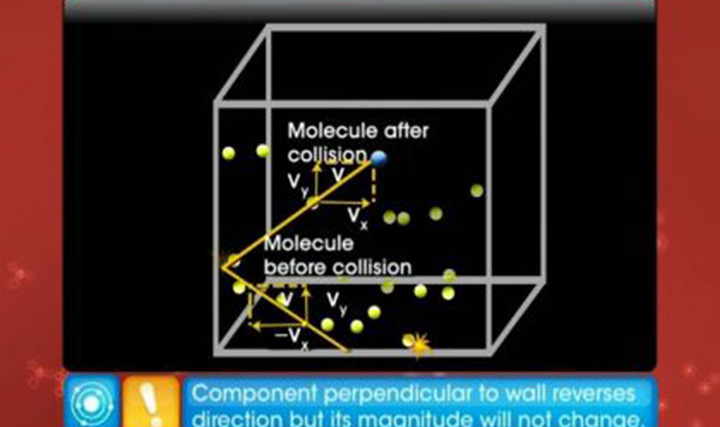
CBSE Class 11-science Physics Kinetic Theory of Gases
- an ideal gas is enclosed in a cylinder at pressure 2 atm and temperature 300k the mean time between two successive collisions is 6×10–?s if the pressure is doubled and temperature is increased to 500k the mean time between two successive collisions will be close to
- Derive allotropic constant in terms of degree of freedom and hence find it's value for monotonic diatomic and polyatomic gases . 4. Derive the expression for mean free path for a gas .
- Kinetic and calculus method
-
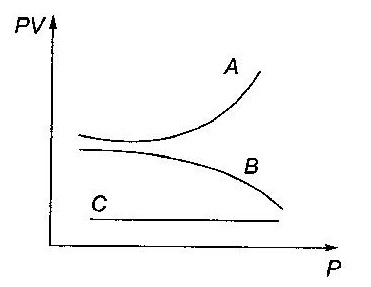
The graph shows the variation of PV with P of given masses of three gases A, B and C. The temperature is kept constant. Which of these is ideal graph?
- Is the r.m.s speed same as the average speed?
- When a gas is suddenly compressed, its temperature rises. Why?
- The volume of a gas sample is increased. Why does the pressure, which is exerted by the gas, decrease?
- In terms of the kinetic theory of gases, explain why the pressure of a gas in a closed container increases when the gas is heated?
- If three molecules have velocities 0.5, 1 and 2 km/s respectively, calculate the ratio of the root mean square speed and average speed.
- Calculate the root mean square velocity of oxygen molecules at 27oC. Take the density of oxygen to be 0.001424 g/cc.