CBSE Class 11-science Answered
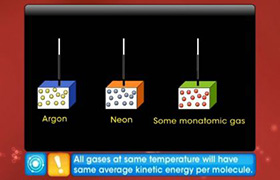
Using the law of equipartition of energy, calculate the total energy of one mole of monoatomic, diatomic and triatomic gases?
Asked by Topperlearning User | 11 May, 2015, 14:26: PM
According to the law of equipartition of energy, the energy is equally distributed among all the degrees of freedom and that is equal to 1/2 kT. Therefore, if the degrees of freedom of gas molecules are f then internal energy of 1 mole of gas will be
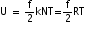 , where N is the Avogadro number.
, where N is the Avogadro number.
|
Atomicity |
Degrees of freedom |
U |
|
Monoatomic gas |
3 |
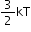 |
|
Diatomic gas |
5 |
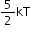 |
|
Angular triatomic gas |
6 |
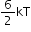 |
|
Linear triatomic gas |
7 |
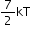 |
Answered by | 11 May, 2015, 16:26: PM
Concept Videos
CBSE 11-science - Physics
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 11-science - Physics
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 11-science - Physics
Asked by Topperlearning User | 11 May, 2015, 13:59: PM
CBSE 11-science - Physics
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 11-science - Physics
Asked by Topperlearning User | 11 May, 2015, 14:13: PM
CBSE 11-science - Physics
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 11-science - Physics
Asked by Topperlearning User | 11 May, 2015, 14:26: PM
CBSE 11-science - Physics
Asked by Topperlearning User | 11 May, 2015, 14:30: PM
CBSE 11-science - Physics
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 11-science - Physics
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM