CBSE Class 12-science Answered
Write the electronic configuration of the following elements of lanthanoid and their possible oxidation state other than +3: (a) Eu and (b) Yb.
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
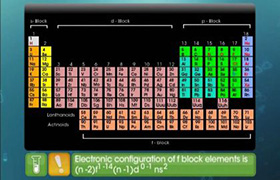
(a) Eu (63): 4f76s2 the possible oxidation state other than +3 is +2 having 4f7 configuration.
(b) Yb (70): 4f146s2 the possible oxidation state other than +3 is +2 having 4f14 configuration.
Answered by | 04 Jun, 2014, 15:23: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by asikmostafamondal8 | 02 May, 2021, 09:08: AM
CBSE 12-science - Chemistry
Asked by ndevi1234 | 11 Mar, 2019, 05:30: AM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 06 Jun, 2016, 14:19: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 27 Jun, 2014, 14:42: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 27 Jun, 2014, 15:34: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM