CBSE Class 12-science Answered
Why the given tautomer is not right ?
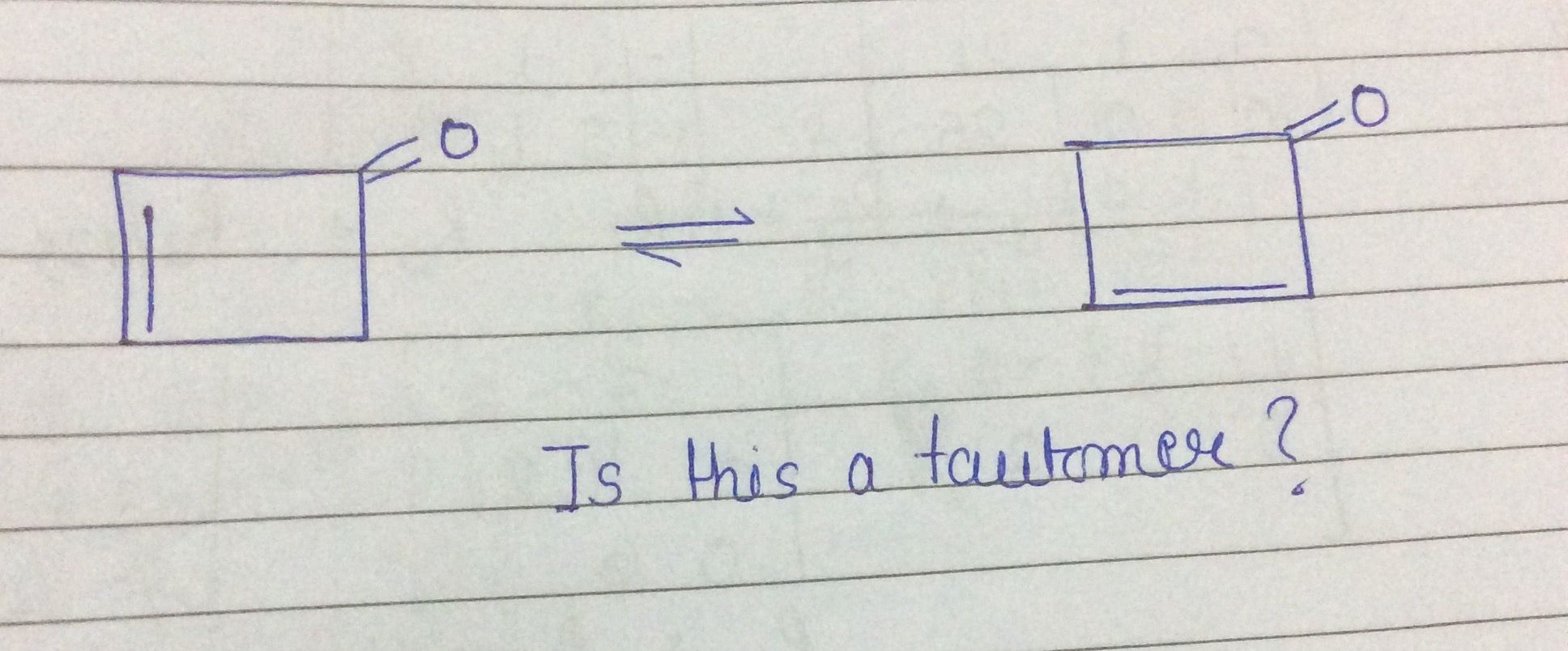
Asked by govtsecschoolnayaganv051 | 28 Aug, 2019, 19:45: PM
As given,

You can see in the given compound the double bond is shifted and formed the same compound just by changing the orientation.
Hence, the above conversion is an example of isomers not of tautomers.
The keto-enol tautomers of given compounds are as follows:

Answered by Ramandeep | 29 Aug, 2019, 10:36: AM
Concept Videos
CBSE 12-science - Chemistry
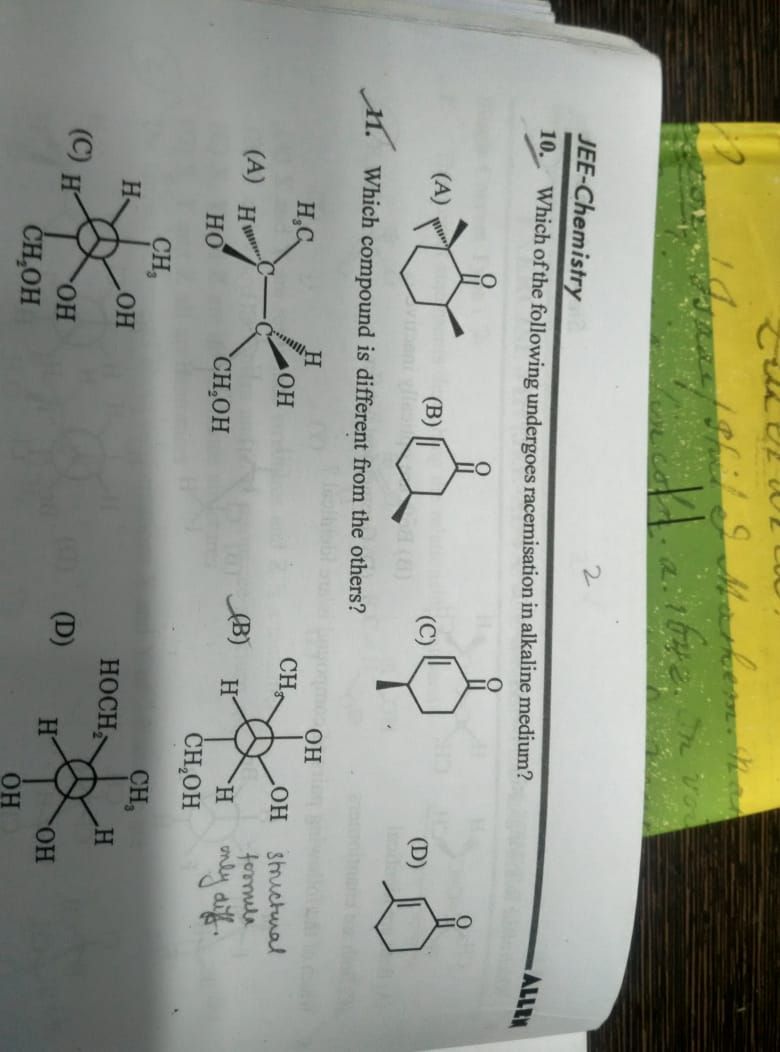
Asked by kaziryan.05 | 29 Jun, 2021, 08:36: AM
CBSE 12-science - Chemistry
Asked by mahaynoorf | 17 Oct, 2020, 20:39: PM
CBSE 12-science - Chemistry
Asked by mufeedatvp2000 | 14 Apr, 2020, 22:58: PM
CBSE 12-science - Chemistry
Asked by mufeedatvp2000 | 14 Apr, 2020, 11:54: AM
CBSE 12-science - Chemistry
Asked by govtsecschoolnayaganv051 | 28 Aug, 2019, 19:45: PM
CBSE 12-science - Chemistry
Asked by dineshchem108 | 20 May, 2019, 23:46: PM
CBSE 12-science - Chemistry
Asked by shobhit | 21 Feb, 2019, 23:01: PM
CBSE 12-science - Chemistry
Asked by Atulcaald | 16 May, 2018, 14:43: PM
CBSE 12-science - Chemistry
Asked by Atulcaald | 16 May, 2018, 14:41: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 08 Apr, 2014, 08:33: AM




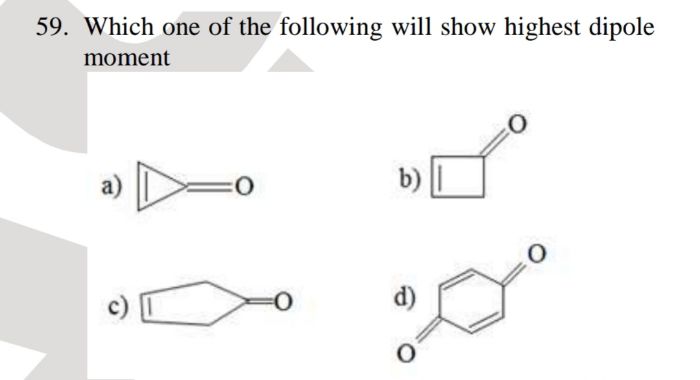
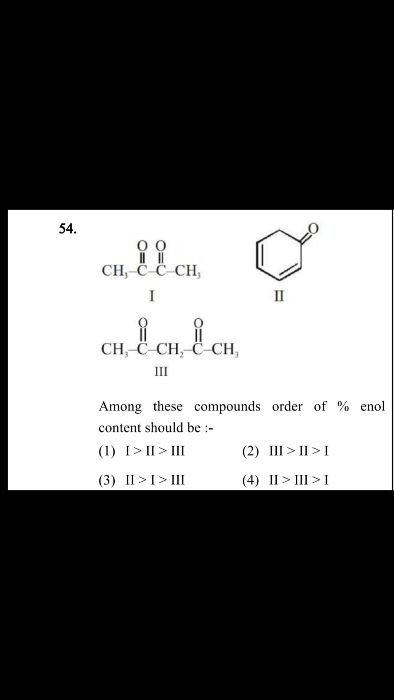
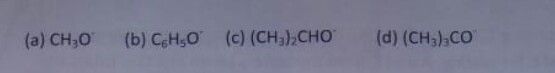
 Y reacts with Z to give
(1)
Y reacts with Z to give
(1)
 (2)
(2)
 (3)
(3)
 (4)
(4)

 Z is :-
(1)
Z is :-
(1)
 (2)
(2)
 (3)
(3)
 (4)
(4)

