CBSE Class 11-science Answered
why nitrogen have different structure of molecular orbital theory
Asked by vermaarti729 | 28 Feb, 2019, 20:28: PM
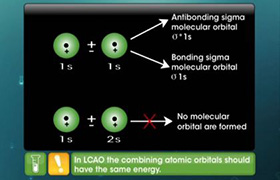
The combination of atomic orbitals takes place due to orbital-orbital interactions is called as s-p mixing.
Due to these interactions, the energy level diagram gets modified. In the case of nitrogen, the energy difference between 2s and 2p levels is quite small.
Due to the close proximity of 2s and 2p orbitals, the![]() orbitals undergo mixing interactions in view of which the energy of
orbitals undergo mixing interactions in view of which the energy of![]() orbitals is raised and it becomes greater than
orbitals is raised and it becomes greater than![]() which do not experience these intermixing interactions.
which do not experience these intermixing interactions.
Answered by Ramandeep | 01 Mar, 2019, 11:13: AM
Concept Videos
CBSE 11-science - Chemistry
Asked by thesouro007 | 20 Mar, 2024, 06:05: AM
CBSE 11-science - Chemistry
Asked by shabnamaijaz83 | 19 Jun, 2022, 10:08: AM
CBSE 11-science - Chemistry
Asked by abnarsale | 31 Dec, 2021, 10:41: AM
CBSE 11-science - Chemistry
Asked by defence | 17 Feb, 2020, 17:09: PM
CBSE 11-science - Chemistry
Asked by mandriosa67 | 13 Feb, 2020, 15:03: PM
CBSE 11-science - Chemistry
Asked by Shrivatsa | 25 Aug, 2019, 14:11: PM
CBSE 11-science - Chemistry
Asked by sonkarshiva009 | 13 Mar, 2019, 17:47: PM
CBSE 11-science - Chemistry
Asked by vermaarti729 | 28 Feb, 2019, 20:28: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM



 4.IF7
5.
4.IF7
5. B.Explain anti bonding and bonding hybridised orbitals.
C._________on hydrolysis gives ethyne while ______ on hydrolysis gives methane.
D.Explain why the colour of Bayer's reagent gets discharged when treated with an alkene.
E.i) State and explain Le Chatelier’s principle. On the basis of this principle discuss the conditions for obtaining the maximum yield of SO3 in the following reaction. 2SO2(g)+ O2(g)?2SO3(g); ?
B.Explain anti bonding and bonding hybridised orbitals.
C._________on hydrolysis gives ethyne while ______ on hydrolysis gives methane.
D.Explain why the colour of Bayer's reagent gets discharged when treated with an alkene.
E.i) State and explain Le Chatelier’s principle. On the basis of this principle discuss the conditions for obtaining the maximum yield of SO3 in the following reaction. 2SO2(g)+ O2(g)?2SO3(g); ?