CBSE Class 11-science Answered
Molecular orbital theory explanation
Asked by mandriosa67 | 13 Feb, 2020, 15:03: PM
Molecular Orbital Theory (MOT):
1) Electrons in a molecule are present in various molecular orbitals as the electrons of atoms are present in various atomic orbitals.
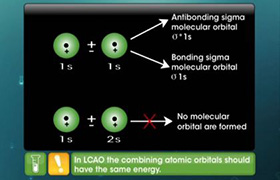
2) Atomic orbitals of comparable energies and proper symmetry combine to form molecular orbitals.
3) While an electron in an atomic orbital is influenced by one nucleus, in a molecular orbital it is influenced by two or more nuclei depending on the number of atoms in the molecule.
Thus, an atomic orbital is monocentric, while a molecular orbital is polycentric.
4) The number of molecular orbitals formed is equal to the number of combining atomic orbitals.
5) When two atomic orbitals combine, two molecular orbitals are formed. One is known as bonding molecular orbital, while the other is called antibonding molecular orbital.
The bonding molecular orbital has lower energy and hence greater stability than the corresponding antibonding molecular orbital.
The bonding molecular orbital has lower energy and hence greater stability than the corresponding antibonding molecular orbital.
6) Just as the electron probability distribution around a nucleus in an atom is given by an atomic orbital, the electron probability distribution around a group of nuclei in a molecule is given by a molecular orbital.
7)The molecular orbitals such as atomic orbitals are filled in accordance with the aufbau principle obeying the Pauli’s exclusion principle and the Hund’s rule.
Answered by Varsha | 14 Feb, 2020, 10:13: AM
Concept Videos
CBSE 11-science - Chemistry
Asked by thesouro007 | 20 Mar, 2024, 06:05: AM
CBSE 11-science - Chemistry
Asked by shabnamaijaz83 | 19 Jun, 2022, 10:08: AM
CBSE 11-science - Chemistry
Asked by abnarsale | 31 Dec, 2021, 10:41: AM
CBSE 11-science - Chemistry
Asked by defence | 17 Feb, 2020, 17:09: PM
CBSE 11-science - Chemistry
Asked by mandriosa67 | 13 Feb, 2020, 15:03: PM
CBSE 11-science - Chemistry
Asked by Shrivatsa | 25 Aug, 2019, 14:11: PM
CBSE 11-science - Chemistry
Asked by sonkarshiva009 | 13 Mar, 2019, 17:47: PM
CBSE 11-science - Chemistry
Asked by vermaarti729 | 28 Feb, 2019, 20:28: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM



 4.IF7
5.
4.IF7
5. B.Explain anti bonding and bonding hybridised orbitals.
C._________on hydrolysis gives ethyne while ______ on hydrolysis gives methane.
D.Explain why the colour of Bayer's reagent gets discharged when treated with an alkene.
E.i) State and explain Le Chatelier’s principle. On the basis of this principle discuss the conditions for obtaining the maximum yield of SO3 in the following reaction. 2SO2(g)+ O2(g)?2SO3(g); ?
B.Explain anti bonding and bonding hybridised orbitals.
C._________on hydrolysis gives ethyne while ______ on hydrolysis gives methane.
D.Explain why the colour of Bayer's reagent gets discharged when treated with an alkene.
E.i) State and explain Le Chatelier’s principle. On the basis of this principle discuss the conditions for obtaining the maximum yield of SO3 in the following reaction. 2SO2(g)+ O2(g)?2SO3(g); ?