CBSE Class 12-science Answered
Why is Aniline very reactive towards bromination ?Why is Aniline very reactive towards bromination ?
Asked by pratikbharadia | 10 Dec, 2009, 08:34: AM
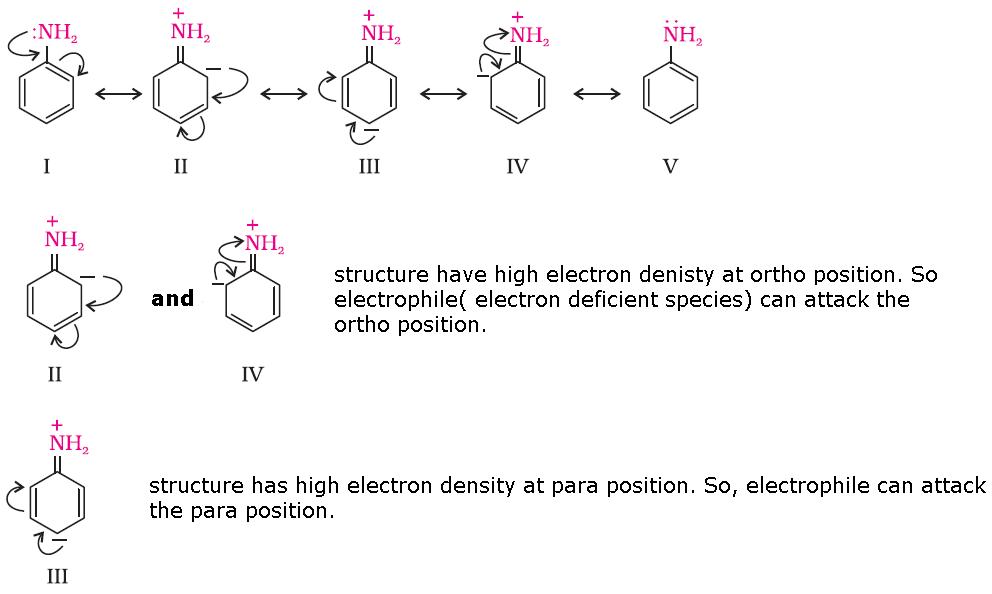
This is because NH2 group in aniline is highly activating group. A lone pair of electrons is present on N which releases the electron density to the benzene ring and hence activates the benzene ring towards electrophilic substitution reactions at ortho and para positions.
Since in bromination, Br+ is the electrophile. So, aniline reacts with bromine water at room temperature to give a white precipitate of 2,4,6 - tribromoaniline.
Answered by | 10 Dec, 2009, 09:54: AM
Concept Videos
CBSE 12-science - Chemistry
Asked by hanihope27 | 01 Mar, 2024, 08:33: PM
CBSE 12-science - Chemistry
Asked by priyankapaliwal255 | 23 Sep, 2023, 05:46: AM
CBSE 12-science - Chemistry
Asked by shwetayaligar205 | 07 Jul, 2022, 08:13: PM
CBSE 12-science - Chemistry
Asked by kaziryan.05 | 06 Jul, 2021, 11:31: PM
CBSE 12-science - Chemistry
Asked by dhivagar25375 | 12 Aug, 2020, 08:34: PM
CBSE 12-science - Chemistry
Asked by danapalanandhan | 28 Jul, 2020, 11:48: AM
CBSE 12-science - Chemistry
Asked by sulaikhasulu393 | 27 May, 2020, 03:34: PM
CBSE 12-science - Chemistry
Asked by mufeedatvp2000 | 15 Apr, 2020, 01:35: PM