CBSE Class 11-science Answered
Why do only some gases and halogens form homoatomic molecules whereas other non-metals don't?
Asked by joykphukan | 14 Sep, 2014, 10:56: AM
Dear joykphukan@gmail.com
Thanks for asking us a question in Ask the Expert section of TopperLearning.com.
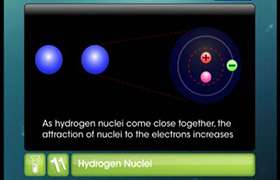
In a homoatomic bond the electrons in the covalent bond are shared between the two nuclei. This means there is no permanent charge separation. This suggest that homoatomic bond will get formed between the some elements of first two rows and halogens.
Regards
Topperlearning Team.
Answered by Arvind Diwale | 14 Sep, 2014, 11:18: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by visalvinod06 | 23 Jun, 2022, 07:39: AM
CBSE 11-science - Chemistry
Asked by bhagwatkrutika6 | 22 Jun, 2022, 09:53: PM
CBSE 11-science - Chemistry
Asked by naveenbahuguna05 | 11 Dec, 2021, 03:19: PM
CBSE 11-science - Chemistry
Asked by akhileshpandeypandey456 | 12 Aug, 2021, 11:09: PM
CBSE 11-science - Chemistry
Asked by toshan.dandi5 | 24 Feb, 2021, 09:24: PM
CBSE 11-science - Chemistry
Asked by patilanil3766 | 01 Jan, 2021, 10:28: PM
CBSE 11-science - Chemistry
Asked by anjalikri2201 | 08 Aug, 2020, 11:22: AM
CBSE 11-science - Chemistry
Asked by khushipatel2124 | 07 Jul, 2020, 04:23: PM
CBSE 11-science - Chemistry
Asked by saurabhchaddha4205 | 03 Jun, 2020, 04:22: PM
CBSE 11-science - Chemistry
Asked by utkarshnikam85.tl | 06 Apr, 2020, 12:13: PM