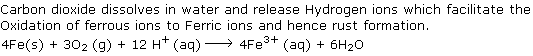CBSE Class 12-science Answered
Why carbon dioxide dissolved in water increases the rate of rusting?
Asked by Topperlearning User | 25 Jun, 2014, 08:36: AM
Answered by | 25 Jun, 2014, 10:36: AM
Concept Videos
CBSE 12-science - Chemistry
Asked by sy985326 | 21 Feb, 2024, 04:23: AM
CBSE 12-science - Chemistry
Asked by rishitadekaraja123 | 07 Feb, 2024, 08:41: AM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 25 Jun, 2014, 09:50: AM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 22 Jun, 2016, 15:00: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 25 Jun, 2014, 09:52: AM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 25 Jun, 2014, 08:36: AM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 25 Jun, 2014, 08:39: AM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 22 Jun, 2016, 15:06: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM