CBSE Class 12-science Answered
which one is more acidic, phenol, carboxylic acid or benzoic acid, why?
Asked by | 28 Dec, 2012, 03:53: PM
Phenols are less (pKa»10) acidic than carboxylic acids (pKa»5)
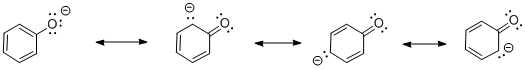
Figure 1. The anion resulting from deprotonation of phenol.

Figure 2. The anion resulting from deprotonation of benzoic acid.
- In phenol, the anion formed by loss of a proton is delocalized. Resonance structures show the negative charge can be shared between the oxygen atom and three of the carbons in the benzene ring.
- In benzoic acid, the anion is also delocalized. This time the negative charge is shared between two different oxygen atoms.
Answered by | 28 Dec, 2012, 04:17: PM
Application Videos
Concept Videos
CBSE 12-science - Chemistry
Asked by kavitabawane190 | 08 Mar, 2024, 05:24: PM
CBSE 12-science - Chemistry
Asked by rp0055293 | 07 Feb, 2024, 08:28: AM
CBSE 12-science - Chemistry
Asked by sanyaagrahari8888 | 28 Nov, 2023, 01:07: PM
CBSE 12-science - Chemistry
Asked by bithikapurkait09052005 | 24 Jun, 2022, 08:35: AM
CBSE 12-science - Chemistry
Asked by rohanm | 14 Feb, 2022, 04:49: PM
CBSE 12-science - Chemistry
Asked by vipulverma | 14 Feb, 2022, 04:44: PM
CBSE 12-science - Chemistry
Asked by kaziryan.05 | 23 Jun, 2021, 08:02: PM
CBSE 12-science - Chemistry
Asked by deepakatur454 | 28 Nov, 2020, 04:29: PM
CBSE 12-science - Chemistry
Asked by holebasubudihal | 09 Aug, 2020, 02:58: PM
CBSE 12-science - Chemistry
Asked by sulaikhasulu393 | 12 Jun, 2020, 09:34: PM











