CBSE Class 11-science Answered
When 100 ml of M/10 H2SO4 is mixed with 500ml of M/10 NaOH,then what is the nature of the resulting solution and the normality of the excess reactant left?
Asked by pb_ckt | 19 May, 2019, 11:57: PM
Gram equivalents of H2SO4 =
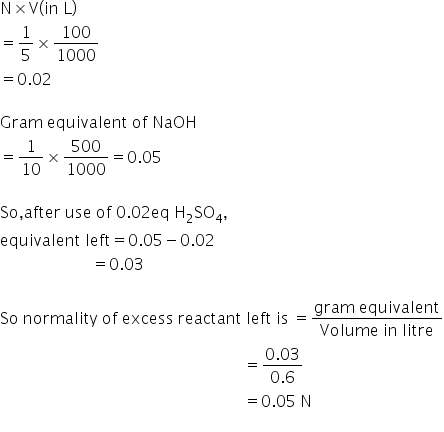
Answered by Ravi | 20 May, 2019, 12:26: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by hm6561889 | 15 Apr, 2024, 07:45: AM
CBSE 11-science - Chemistry
Asked by ansh.skulkarni1158 | 07 Apr, 2024, 11:03: AM
CBSE 11-science - Chemistry
Asked by VarunTYAGi9013 | 21 Oct, 2023, 07:14: PM
CBSE 11-science - Chemistry
Asked by pujakum60022 | 11 Jun, 2023, 08:09: PM
CBSE 11-science - Chemistry
Asked by vibhutimandal70 | 26 Jun, 2022, 10:39: PM
CBSE 11-science - Chemistry
Asked by sd8022567 | 10 Jun, 2022, 11:39: PM
CBSE 11-science - Chemistry
Asked by Bhathika1434 | 13 Oct, 2020, 07:13: PM
CBSE 11-science - Chemistry
Asked by tiparsemahesh | 04 Oct, 2020, 07:35: PM
CBSE 11-science - Chemistry
Asked by nirajrane1234 | 09 Sep, 2020, 09:53: PM
CBSE 11-science - Chemistry
Asked by Ssajal020 | 25 Aug, 2020, 06:14: PM






