CBSE Class 12-science Answered
What is the formula of a compound in which the element Y forms hcp lattice
and atoms of X occupy 2/3rd of tetrahedral voids
Asked by upasana.sobti | 16 Jan, 2019, 13:59: PM
The no. of atoms in hcp lattice, Y = 6
Tetrahedral void = 2× 6
= 12
2/3 of these voids are occupied by X,
Therefore,
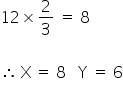
So the formula of a compound formed is X4Y3.
Answered by Varsha | 16 Jan, 2019, 15:52: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by rasmimajhi07 | 21 Nov, 2023, 22:22: PM
CBSE 12-science - Chemistry
Asked by pradeepkumar70258 | 04 Oct, 2023, 22:30: PM
CBSE 12-science - Chemistry
Asked by akash9322793205 | 12 Aug, 2021, 09:30: AM
CBSE 12-science - Chemistry
Asked by thakurranjan54 | 08 Feb, 2021, 18:32: PM
CBSE 12-science - Chemistry
Asked by sildasholly2002 | 26 Jul, 2020, 18:37: PM
CBSE 12-science - Chemistry
Asked by tejuaaygole | 11 Jul, 2020, 10:40: AM
CBSE 12-science - Chemistry
Asked by chandlerbong164 | 12 Apr, 2020, 19:22: PM
CBSE 12-science - Chemistry
Asked by mangeshkale423 | 25 Feb, 2020, 17:12: PM
CBSE 12-science - Chemistry
Asked by upasana.sobti | 16 Jan, 2019, 13:59: PM




