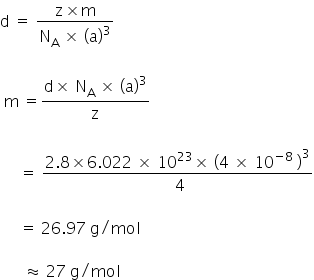CBSE Class 12-science Answered
Q.1 What is the formula of a compound in which the element Y forms hcp lattice and atoms of XE occupy 2/3rd of tetrahedral voids?
Element Y forms hcp lattice,
No. of atoms of Y in hcp = 6
No. of tetrahedral voids, X = 2 × 6 = 12
Only 2/3rd of these are ocuppied by X so,
12×2/3= 8
So, X = 8 and Y = 6
So the formula of the compound formed is X4Y3.
Q.2 An element with density 2.8g cm-3 forms a fcc unit cell with edge length 4 × l0-8cm. Calculate the molar mass of the element.[2M](Given: NA=6.022 × 1023 mol-1)
Edge length a = 4 × 10-8 cm
Density d, = 2.8 g cm-3
As the lattice is fcc type, the no. of atoms per unit cell, z = 4
NA=6.022 × 1023 mol-1