CBSE Class 12-science Answered
each edge of a cubic unit cell is 400 pm long. if atomic weight of the element is 120 and its density is 6.25 g/cm3. The crystal lattice is :
a) simple cubic b)BCC
c) FCC c) NONE
Asked by chandlerbong164 | 12 Apr, 2020, 19:22: PM
Option (b) is correct.
The crystal lattice is bcc.
Given:
Atomic weight, M = 120
NA = 6.022 × 1023
Unit cell, a = 400 pm
= 400 × 10-10
We have,
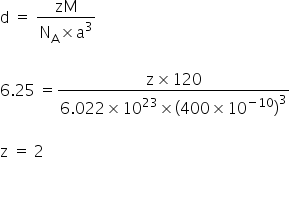
Answered by Varsha | 13 Apr, 2020, 14:14: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by rasmimajhi07 | 21 Nov, 2023, 22:22: PM
CBSE 12-science - Chemistry
Asked by pradeepkumar70258 | 04 Oct, 2023, 22:30: PM
CBSE 12-science - Chemistry
Asked by akash9322793205 | 12 Aug, 2021, 09:30: AM
CBSE 12-science - Chemistry
Asked by thakurranjan54 | 08 Feb, 2021, 18:32: PM
CBSE 12-science - Chemistry
Asked by sildasholly2002 | 26 Jul, 2020, 18:37: PM
CBSE 12-science - Chemistry
Asked by tejuaaygole | 11 Jul, 2020, 10:40: AM
CBSE 12-science - Chemistry
Asked by chandlerbong164 | 12 Apr, 2020, 19:22: PM
CBSE 12-science - Chemistry
Asked by mangeshkale423 | 25 Feb, 2020, 17:12: PM
CBSE 12-science - Chemistry
Asked by upasana.sobti | 16 Jan, 2019, 13:59: PM




