CBSE Class 12-science Answered
In a FCC lattice , atoms A occupy the corner positions and atoms B occupy the face
centre positions. If one atom of B is missing from one of the face centred points,
calculate the formula of the compound
Asked by akash9322793205 | 12 Aug, 2021, 09:30: AM
In a face centered cubic lattice, atom (A) occupies the corner positions. There are 8 corner positions and each position contributes one eighth to the unit cell. Hence, total number of (A) atoms per unit cell= 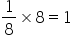
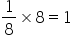
Atom (B) occupied the face centre positions. There are six face centre positions. One atom of (B) is missing from one of the face centered points. Thus, there are 5 face centre positions that are occupied with (B). Each such position contributes one half to the unit cell. Hence, total number of (B) atoms per unit cell.=
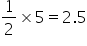
The formula of the compound is AB2.5
A2 B5
Answered by Ravi | 12 Aug, 2021, 12:01: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by rasmimajhi07 | 21 Nov, 2023, 22:22: PM
CBSE 12-science - Chemistry
Asked by pradeepkumar70258 | 04 Oct, 2023, 22:30: PM
CBSE 12-science - Chemistry
Asked by akash9322793205 | 12 Aug, 2021, 09:30: AM
CBSE 12-science - Chemistry
Asked by thakurranjan54 | 08 Feb, 2021, 18:32: PM
CBSE 12-science - Chemistry
Asked by sildasholly2002 | 26 Jul, 2020, 18:37: PM
CBSE 12-science - Chemistry
Asked by tejuaaygole | 11 Jul, 2020, 10:40: AM
CBSE 12-science - Chemistry
Asked by chandlerbong164 | 12 Apr, 2020, 19:22: PM
CBSE 12-science - Chemistry
Asked by mangeshkale423 | 25 Feb, 2020, 17:12: PM
CBSE 12-science - Chemistry
Asked by upasana.sobti | 16 Jan, 2019, 13:59: PM




