CBSE Class 11-science Answered
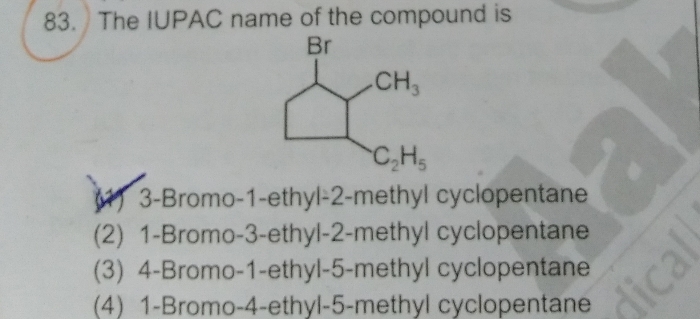
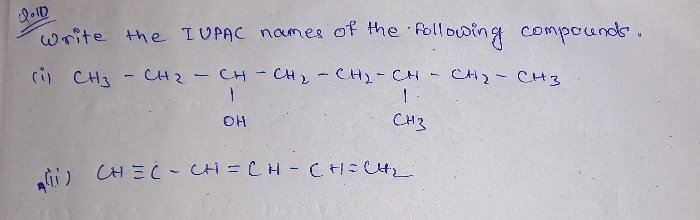
what is the difference between electron withdrawing and electron donating?
Asked by pooja dev | 23 Jan, 2011, 12:00: AM
Dear Student
Electron donating groups as the name suggests are the groups of atoms that can donate an electron or electron pairs.
These are the groups rich in electron that can give out one or more electrons to form a bond. Electron donating groups list comprises of the groups that can donate electrons. examples are: alkyl groups (CH3, C2H5, etc.), amino group, etc.
An electron withdrawing group or EWG draws electrons away from a reaction center. When this center is an electron rich carbanion or an alkoxide anion with the presence of the substituent that has a stabilizing effect. Examples of such groups are halogens (F, Cl), nitriles CN;carbonyls CO;nitro groups NO2.
We hope that clarifies your query.
Regards
Team
Topperlearning
Answered by | 23 Jan, 2011, 11:11: AM
Concept Videos
CBSE 11-science - Chemistry
Asked by josephineanto1960 | 28 Mar, 2024, 12:50: PM
CBSE 11-science - Chemistry
Asked by neet2025targetgo | 25 Mar, 2024, 10:13: AM
CBSE 11-science - Chemistry
Asked by ap4450962 | 12 Mar, 2024, 07:35: PM
CBSE 11-science - Chemistry
Asked by pamjat.8888 | 31 Jan, 2024, 11:31: AM
CBSE 11-science - Chemistry
Asked by sahumahesh3973 | 20 Jan, 2024, 06:33: PM
CBSE 11-science - Chemistry
Asked by aswintj2007 | 07 Jan, 2024, 08:53: PM
CBSE 11-science - Chemistry
Asked by dipalisingh0908 | 05 Nov, 2023, 02:24: PM
CBSE 11-science - Chemistry
Asked by badalbehera258369 | 19 Oct, 2023, 02:01: PM
CBSE 11-science - Chemistry
Asked by prakrutikhosla | 16 Sep, 2023, 06:31: PM
CBSE 11-science - Chemistry
Asked by shahintkjnv2016 | 13 Jun, 2022, 07:17: PM