CBSE Class 11-science Answered
what is the difference between avg kinetic energy and root mean square velocity?
Asked by n jain | 17 Mar, 2011, 12:00: AM
Dear student,
Root mean square velocity: The square root of the average of the squares of the individual velocities of the gas particles in a mixture. To put it in a simpler way , it's the average of how fast the particles in a gas are going (assuming you ignore the direction they're traveling in).
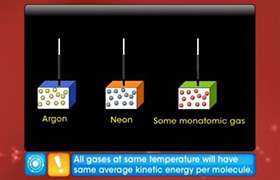
Average kinetic energy:
- kinetic energy of one molecule: ½ mv2
- average kinetic energy of one mole of molecules ½ M(vrms)2 = (3/2) RT
- average molecular kinetic energy depends only on temperature for ideal gases
Hope this helps.
Thankyou
Team
Topperlearning.com
Answered by | 17 Mar, 2011, 01:11: PM
Concept Videos
CBSE 11-science - Physics
Asked by ifrayaseen31 | 28 Oct, 2023, 09:26: AM
CBSE 11-science - Physics
Asked by pratikshyadashrkl | 01 May, 2020, 10:24: AM
CBSE 11-science - Physics
Asked by karanchandra34 | 29 Jan, 2019, 11:10: PM
CBSE 11-science - Physics
Asked by Madhurimaurya609 | 11 Jul, 2018, 08:14: PM
CBSE 11-science - Physics
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 11-science - Physics
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 11-science - Physics
Asked by Topperlearning User | 11 May, 2015, 01:59: PM
CBSE 11-science - Physics
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 11-science - Physics
Asked by Topperlearning User | 11 May, 2015, 02:13: PM
CBSE 11-science - Physics
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM