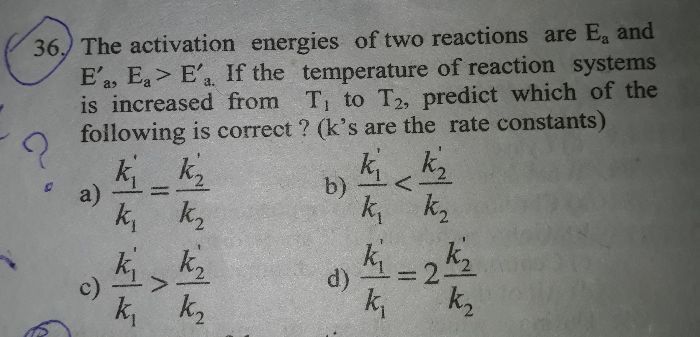CBSE Class 12-science Answered
what is effect of temperature on activation energy?
Asked by | 21 Feb, 2008, 04:59: PM
Ea is activation energy the threshold required for reaction to occur. With increase in energy the kinetic energy increases the rate constant increses exponentialyas per arrhenius equation. rate constant k  e-Ea/RTso Ea doenot change with temperature. It is equal to kinetic energy possesed by avagadro number of molecules.
e-Ea/RTso Ea doenot change with temperature. It is equal to kinetic energy possesed by avagadro number of molecules.
Answered by | 07 Dec, 2017, 06:48: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by bhadauriyax | 30 Nov, 2023, 06:23: PM
CBSE 12-science - Chemistry
Asked by rahulbiswal946 | 08 Nov, 2023, 07:01: PM
CBSE 12-science - Chemistry
Asked by arshbhatia0809 | 22 Jul, 2021, 09:47: PM
CBSE 12-science - Chemistry
Asked by Surendersingh0493 | 18 Oct, 2020, 02:05: PM
CBSE 12-science - Chemistry
Asked by khandarev3580 | 10 Oct, 2020, 10:54: AM
CBSE 12-science - Chemistry
Asked by dr.akanksha0411 | 07 Aug, 2020, 11:56: AM
CBSE 12-science - Chemistry
Asked by amritha2960 | 13 May, 2020, 08:26: AM
CBSE 12-science - Chemistry
Asked by sulaikhasulu393 | 02 May, 2020, 09:20: AM
CBSE 12-science - Chemistry
Asked by leelakrishnapallapotu143 | 29 Mar, 2020, 07:27: PM
CBSE 12-science - Chemistry
Asked by lovemaan5500 | 30 Jan, 2020, 03:29: PM