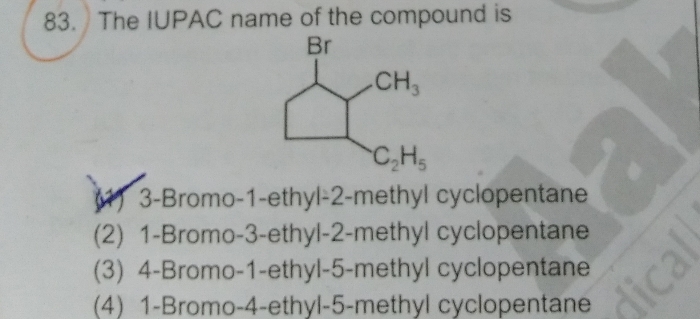
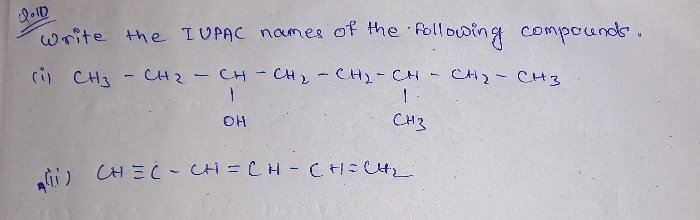
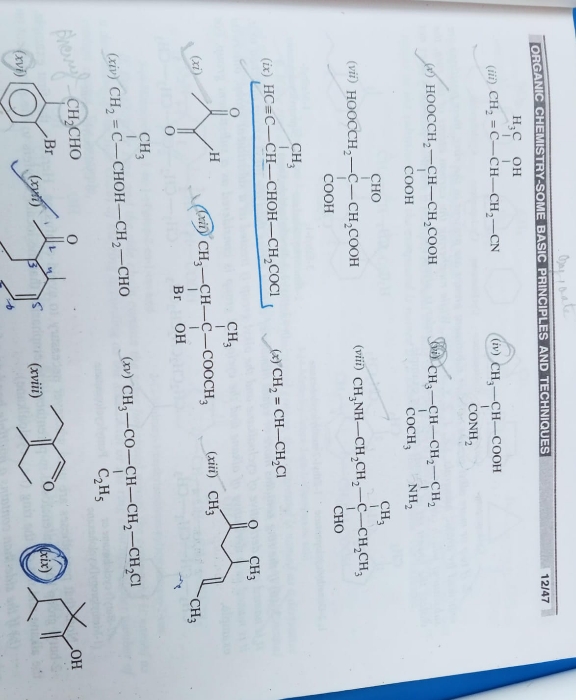
CBSE Class 11-science Answered
what are Fischer projections?How will we draw these structures?
Asked by | 29 Feb, 2008, 05:30: PM
The Fischer Projection represents every stereocenter as a cross. The horizontal line represents bonds extending out of the plane of the page, whereas the vertical line represents bonds extending into the plane of the page.
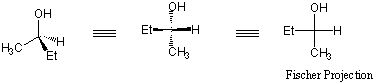
When working with Fischer Projections, keep in mind the following rules:
- Because the "up" and "down" aspects of the bonds don't change, a Fischer projection may be rotated by 180 degrees without changing its meaning.
- A Fischer projection may not be rotated by 90 degrees. Such a rotation typically changes the configuration to the enantiomer.
- To find the enantiomer of a molecule drawn as a Fischer projection, simply exchange the right and left horizontal bonds.
- To determine whether the molecule in Fischer projection is a meso compound, draw a horizontal line through the center of the molecule and determine whether the molecule is symmetric about that line.
Answered by | 29 Feb, 2008, 05:44: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by josephineanto1960 | 28 Mar, 2024, 12:50: PM
CBSE 11-science - Chemistry
Asked by neet2025targetgo | 25 Mar, 2024, 10:13: AM
CBSE 11-science - Chemistry
Asked by ap4450962 | 12 Mar, 2024, 07:35: PM
CBSE 11-science - Chemistry
Asked by pamjat.8888 | 31 Jan, 2024, 11:31: AM
CBSE 11-science - Chemistry
Asked by sahumahesh3973 | 20 Jan, 2024, 06:33: PM
CBSE 11-science - Chemistry
Asked by aswintj2007 | 07 Jan, 2024, 08:53: PM
CBSE 11-science - Chemistry
Asked by dipalisingh0908 | 05 Nov, 2023, 02:24: PM
CBSE 11-science - Chemistry
Asked by badalbehera258369 | 19 Oct, 2023, 02:01: PM
CBSE 11-science - Chemistry
Asked by prakrutikhosla | 16 Sep, 2023, 06:31: PM
CBSE 11-science - Chemistry
Asked by shahintkjnv2016 | 13 Jun, 2022, 07:17: PM