CBSE Class 12-science Answered
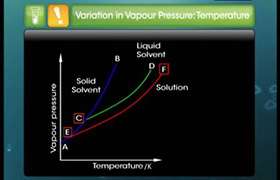
Vapour Pressure
Asked by | 14 Apr, 2008, 11:34: AM
As the in volitile solid particles interupt the evaporation the VP decreases.
Answered by | 21 Dec, 2017, 01:04: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by varinder2149 | 10 Dec, 2023, 08:21: PM
CBSE 12-science - Chemistry
Asked by vekariyaparth61 | 16 May, 2022, 04:33: PM
CBSE 12-science - Chemistry
Asked by goyalpavitarta | 03 May, 2021, 01:27: PM
CBSE 12-science - Chemistry
Asked by goyalpavitarta | 03 May, 2021, 01:25: PM
CBSE 12-science - Chemistry
Asked by goyalpavitarta | 03 May, 2021, 01:20: PM
CBSE 12-science - Chemistry
Asked by sshashu993 | 25 Jul, 2020, 08:02: AM
CBSE 12-science - Chemistry
Asked by anukritisingh8103.bmps | 15 Jul, 2020, 05:52: PM
CBSE 12-science - Chemistry
Asked by sharmasherryal | 25 May, 2020, 09:54: AM
CBSE 12-science - Chemistry
Asked by panthpreet0221 | 06 May, 2020, 10:41: AM