CBSE Class 12-science Answered
Using de Boglie's hypothesis, explain with the help of a suitable diagram, Bohr's second postulate of quantisation of energy levels in a hydrogen atom.
Asked by rohitraman1115 | 14 Jan, 2019, 12:02: PM
De broglie assumed that electrons move in circular orbit with it mean position of vibration at periphery of assumed orbit. So assuming electron move like wave up down on a circular orbit we can say total periphery of that circular orbit will be equal to integral times of wavelength of vibration that is n×λ
Eqauting this we get n×λ = 2πr , where r is the radius of desire orbit
So λ= 2πr/n
Again as we know λ = h/p , where p is liner momentum (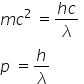 )
)
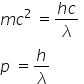 )
)From these two equation we can get the value of radius
And once value of radius is known we can find the energy of the orbit with desire radius from cuolombs law of electrostatic.
For diagram please refer NCERT book.
Answered by Ankit K | 14 Jan, 2019, 22:31: PM
CBSE 12-science - Physics
Asked by dasrituparna1999 | 12 Apr, 2024, 21:26: PM
CBSE 12-science - Physics
Asked by Akshatkuma2004 | 04 Jul, 2021, 16:04: PM
CBSE 12-science - Physics
Asked by rsrakesh932 | 12 Jun, 2020, 16:38: PM
CBSE 12-science - Physics
Asked by yadavnitish688 | 14 Dec, 2019, 06:04: AM
CBSE 12-science - Physics
Asked by sidiz.shrestha07 | 26 May, 2019, 06:42: AM
CBSE 12-science - Physics
Asked by pardeepkumar2281 | 28 Feb, 2019, 19:24: PM
CBSE 12-science - Physics
Asked by pradeepjsme | 30 Jan, 2019, 15:54: PM
CBSE 12-science - Physics
Asked by pradeepjsme | 30 Jan, 2019, 15:51: PM
CBSE 12-science - Physics
Asked by rohitraman1115 | 14 Jan, 2019, 12:02: PM
CBSE 12-science - Physics
Asked by rohitraman1115 | 14 Jan, 2019, 12:01: PM

