CBSE Class 12-science Answered
The number of moles in an elemental sample is just the ratio of the mass m to atomic mass 4.
n=
A
The number of protons N, in an elemental sample is Z (atomic number) times the number of moles of Avogadro's number NA N=ZnN P A Copper has an atomic mass of 63.5 g/mole and 29 protons per atom. If such an atom were neutral, then it would have as many protons as electrons. If you remove electrons from a neutral state, then you get a net positive charge.
Given a 54 gram ball of copper with a net charge of 2.2 µC, what fraction of the copper's electrons have been removed?
Asked by dasrituparna1999 | 12 Apr, 2024, 21:26: PM
mass of copper ball = 54 gram
1 mole of copper ball = 63.5 gram
number of moles in the copper ball = ( 54/63.5 ) = 0.850
Number of atoms in copper ball is produce of number of moles and Avagadro number N = 6.022 × 1023 .
Number of atoms in copper ball = 0.850 × 6.022 × 1023
If each atom has 29 protons, then number of electrons per atom is 29 .
Total number of electrons in copper ball, Ne = 0.850 × 29 × 6.022 × 1023 ..................... (1)
If the charge on copper ball is 2.2 μC and charge of electron is -1.602 × 10-19 C ,
then number of electrons removed ne is
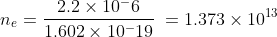
Fraction of electron removed from copper ball is
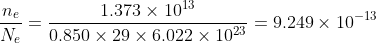
Answered by Thiyagarajan K | 12 Apr, 2024, 22:10: PM
CBSE 12-science - Physics
Asked by dasrituparna1999 | 12 Apr, 2024, 21:26: PM
CBSE 12-science - Physics
Asked by Akshatkuma2004 | 04 Jul, 2021, 16:04: PM
CBSE 12-science - Physics
Asked by rsrakesh932 | 12 Jun, 2020, 16:38: PM
CBSE 12-science - Physics
Asked by yadavnitish688 | 14 Dec, 2019, 06:04: AM
CBSE 12-science - Physics
Asked by sidiz.shrestha07 | 26 May, 2019, 06:42: AM
CBSE 12-science - Physics
Asked by pardeepkumar2281 | 28 Feb, 2019, 19:24: PM
CBSE 12-science - Physics
Asked by pradeepjsme | 30 Jan, 2019, 15:54: PM
CBSE 12-science - Physics
Asked by pradeepjsme | 30 Jan, 2019, 15:51: PM
CBSE 12-science - Physics
Asked by rohitraman1115 | 14 Jan, 2019, 12:02: PM
CBSE 12-science - Physics
Asked by rohitraman1115 | 14 Jan, 2019, 12:01: PM

