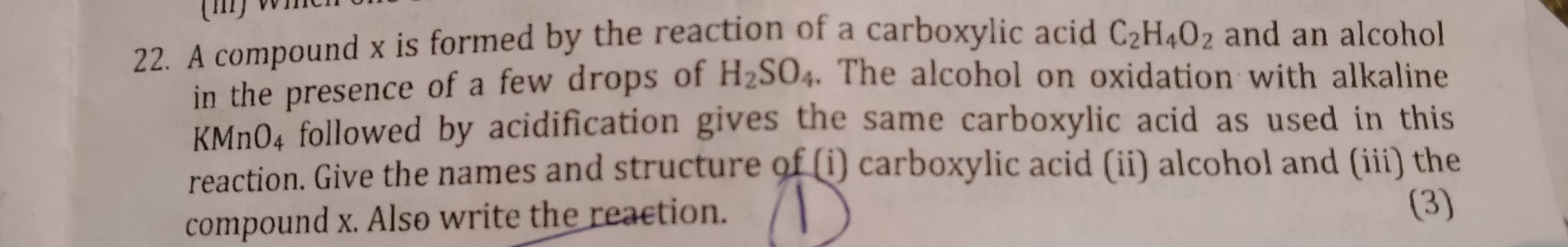CBSE Class 10 Answered
The general formula of an ester where R represents an alkyl group is ? what is ans and explain?
Asked by vedantsagrawal23 | 05 Dec, 2019, 08:34: AM
Esters have the general formula: RCOOR' where R and R' are alkyl groups. They are formed by the reaction of acids with alcohols.
If the ester is formed from alcohol R'OH and acid RCOOH then the formula of ester will be RCOOR' followed by elimination of a water molecule.
For example: CH3COOCH3 - Methyl ethanoate
CH3COOC2H5 - Ethyl ethanoate
While naming esters, use the alcohol group first, in this case ethyl, then the carboxylic acid name second with the -oate ending, in this case ethanoate.
Some other examples of esters:
Ethyl propanoate is formed from ethanol and propanoic acid therefore its formula is CH3CH2COOCH2CH3
Propyl methanoate is formed from propanol and methanoic acid therefore its formula is HCOOCH2CH2CH3
If the ester is formed from alcohol R'OH and acid RCOOH then the formula of ester will be RCOOR' followed by elimination of a water molecule.
Answered by Ramandeep | 05 Dec, 2019, 10:55: AM
Application Videos
Concept Videos
CBSE 10 - Chemistry
Asked by sneh | 27 Mar, 2020, 10:11: AM
CBSE 10 - Chemistry
Asked by pranjaliinamdar2004 | 29 Feb, 2020, 19:20: PM
CBSE 10 - Chemistry
Asked by sweetykhatri99254 | 27 Feb, 2020, 15:40: PM
CBSE 10 - Chemistry
Asked by priyanshiishu | 30 Jan, 2020, 10:39: AM
CBSE 10 - Chemistry
Asked by kamalnayansingh7 | 13 Jan, 2020, 08:35: AM
CBSE 10 - Chemistry
Asked by Deepak | 22 Dec, 2019, 23:20: PM
CBSE 10 - Chemistry
Asked by ritikraghuwanshi6986 | 16 Dec, 2019, 20:42: PM
CBSE 10 - Chemistry
Asked by vedantsagrawal23 | 05 Dec, 2019, 08:34: AM
CBSE 10 - Chemistry
Asked by aryasaxena2003 | 25 Jul, 2019, 17:35: PM
CBSE 10 - Chemistry
Asked by rushabhjain.avv | 21 Mar, 2019, 22:07: PM