CBSE Class 11-science Answered
The frequency of the radiation emitted when electron falls from infinity to n=1 state for he+ would be
Asked by arushidabhade | 08 Jul, 2019, 20:11: PM
Given:
For He2+
n1 = 1
n2 = ∞
We have,
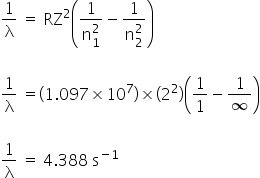
The frequency of the radiation is 4.388 s-1.
Answered by Varsha | 09 Jul, 2019, 10:47: AM
Concept Videos
CBSE 11-science - Chemistry
Asked by ee7511641 | 13 Jan, 2024, 15:37: PM
CBSE 11-science - Chemistry
Asked by shrreya27harshitha | 17 Jul, 2022, 16:15: PM
CBSE 11-science - Chemistry
Asked by murchana10022002 | 10 Aug, 2020, 12:06: PM
CBSE 11-science - Chemistry
Asked by arushidabhade | 08 Jul, 2019, 20:11: PM
CBSE 11-science - Chemistry
Asked by arushidabhade | 29 Jun, 2019, 20:42: PM
CBSE 11-science - Chemistry
Asked by amritchirania | 16 Jun, 2019, 12:31: PM
CBSE 11-science - Chemistry
Asked by arunavamitra50 | 18 Jun, 2018, 18:35: PM
CBSE 11-science - Chemistry
Asked by smanishkumar2002 | 20 May, 2018, 22:46: PM
CBSE 11-science - Chemistry
Asked by smanishkumar2002 | 20 May, 2018, 22:45: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 16 Apr, 2015, 10:00: AM







