CBSE Class 11-science Answered
Find the number of spectral lines obtained when electron de-excites from 5th to the 1st energy level but no line is seen in balmer series ?
Asked by arunavamitra50 | 18 Jun, 2018, 18:35: PM
If the electron jumps from n2 = 5 to n1 = 1 then following are the transitions possible.
- 5→ 4
- 5→ 3
- 5→ 2
- 5 → 1
- 4 → 3
- 4→ 2
- 4→ 1
- 3 → 2
- 3→ 1
- 2 → 1
Hence there are 10 transitions and hence 10 spectral lines possible.
The general formula for the number of spectral lines emitted is
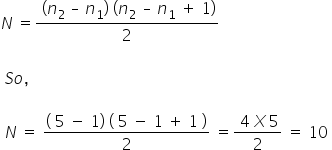
Answered by Ramandeep | 21 Jun, 2018, 14:40: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by ee7511641 | 13 Jan, 2024, 15:37: PM
CBSE 11-science - Chemistry
Asked by shrreya27harshitha | 17 Jul, 2022, 16:15: PM
CBSE 11-science - Chemistry
Asked by murchana10022002 | 10 Aug, 2020, 12:06: PM
CBSE 11-science - Chemistry
Asked by arushidabhade | 08 Jul, 2019, 20:11: PM
CBSE 11-science - Chemistry
Asked by arushidabhade | 29 Jun, 2019, 20:42: PM
CBSE 11-science - Chemistry
Asked by amritchirania | 16 Jun, 2019, 12:31: PM
CBSE 11-science - Chemistry
Asked by arunavamitra50 | 18 Jun, 2018, 18:35: PM
CBSE 11-science - Chemistry
Asked by smanishkumar2002 | 20 May, 2018, 22:46: PM
CBSE 11-science - Chemistry
Asked by smanishkumar2002 | 20 May, 2018, 22:45: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 16 Apr, 2015, 10:00: AM







