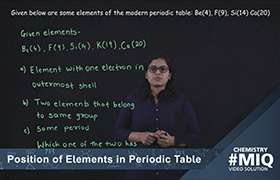
CBSE Class 10 Answered
state 3 reasons why hydrogen can be placed in group 1 or group 17 ?
Asked by | 17 Mar, 2012, 02:36: PM
Resemblence of hydrogen with alkali metals (Group -1)----
(1)electronic configuration :- like alkali metals hydrogen also contains one electron in its outermost shell...
hydrogen's electronic configuration = 1s1
lithium' electronic configuration = 1s2 2s1
sodium's electronic configuration = 1s2 2s2 2p6 3s1
(2)electropositive character:- like alkali metals hydrogen also looses its only one electron to form hydrogen ion ,H(+)
H ----> H(+) + e-
Na ----> Na(+) + e-
thus hydrogen like alkali metals exhibit electropositive character...
(3).oxidation state :-like alkali metals hydrogen exhibits an oxidation state of +1 in its compounds...
for example- HCl ,, NaCl ,, KBr ...here oxidation state of H is same as Na and K i.e. +1..
(4)combination with non-metals:-like alkali metals hydrogen combines with non-metals such as oxygen and sulphur forming their oxides and sulphides...for example-- H2O, like Na2O ,K2O
H2S like Na2S,K2S
(5)Like alkali metals hydrogen also act as a strong reducing agent ..
For example :
Fe3O4 + 4H2 -----> 3Fe + 4H2O
B2O3 + 6K -----> 2B + 3K2O
resempblence with halogens(Group -17 or Group VII)---
(1)electronic configuration:-all halogens have 7 electrons in their repective outermost shell and thus have one less electron than the stable configuration of nearest noble gas ..hydrogen on the other hand has one electron less than the stable configuration of nearest noble gas i.e. Helium ..
(2)electronegative character:-halogens have a strong tendency to gain one electron to form halide ions ..in a similar way hydrogen shows some tendency to gain one electron to form hydride ion ..
H + e- -----> H(-) (He gas configuration)
Cl + e -----> Cl(-) (Ar gas configuration)
(3)ionization energy:-ionization enrgy of hydrogen is similar to that of halogens but much higher than alkali metals..
for example ionizaion energy of H = 1312 kj/mole
ionizaion energy of F(a halogen) = 1681 kj/mole
ionizaion energy of Cl(a halogen) = 1255 kj/mole
ionizaion energy of Na (an alkali metal) = 496 kj/mole
ionizaion energy of K (an alkali metal) = 419 kj/mole
(4)oxidation state :- just like halogens hydrogen shows an oxidation state of -1..
for example -- NaH like NaCl (both hydrogen and chlorine are in -1 oxidation state)
CaH2 like CaCl2 (both hydrogen and chlorine are in -1 oxidation state)
(5)like halogens,, hydrogen easily combines with non metal such as carbon,silicon,nitrogen etc to form covalent compounds ...
with hydrogen : CH4,SiH4,NH3
with Halogens : CCl4,SiCl4,NCl3
(1)electronic configuration :- like alkali metals hydrogen also contains one electron in its outermost shell...
hydrogen's electronic configuration = 1s1
lithium' electronic configuration = 1s2 2s1
sodium's electronic configuration = 1s2 2s2 2p6 3s1
(2)electropositive character:- like alkali metals hydrogen also looses its only one electron to form hydrogen ion ,H(+)
H ----> H(+) + e-
Na ----> Na(+) + e-
thus hydrogen like alkali metals exhibit electropositive character...
(3).oxidation state :-like alkali metals hydrogen exhibits an oxidation state of +1 in its compounds...
for example- HCl ,, NaCl ,, KBr ...here oxidation state of H is same as Na and K i.e. +1..
(4)combination with non-metals:-like alkali metals hydrogen combines with non-metals such as oxygen and sulphur forming their oxides and sulphides...for example-- H2O, like Na2O ,K2O
H2S like Na2S,K2S
(5)Like alkali metals hydrogen also act as a strong reducing agent ..
For example :
Fe3O4 + 4H2 -----> 3Fe + 4H2O
B2O3 + 6K -----> 2B + 3K2O
resempblence with halogens(Group -17 or Group VII)---
(1)electronic configuration:-all halogens have 7 electrons in their repective outermost shell and thus have one less electron than the stable configuration of nearest noble gas ..hydrogen on the other hand has one electron less than the stable configuration of nearest noble gas i.e. Helium ..
(2)electronegative character:-halogens have a strong tendency to gain one electron to form halide ions ..in a similar way hydrogen shows some tendency to gain one electron to form hydride ion ..
H + e- -----> H(-) (He gas configuration)
Cl + e -----> Cl(-) (Ar gas configuration)
(3)ionization energy:-ionization enrgy of hydrogen is similar to that of halogens but much higher than alkali metals..
for example ionizaion energy of H = 1312 kj/mole
ionizaion energy of F(a halogen) = 1681 kj/mole
ionizaion energy of Cl(a halogen) = 1255 kj/mole
ionizaion energy of Na (an alkali metal) = 496 kj/mole
ionizaion energy of K (an alkali metal) = 419 kj/mole
(4)oxidation state :- just like halogens hydrogen shows an oxidation state of -1..
for example -- NaH like NaCl (both hydrogen and chlorine are in -1 oxidation state)
CaH2 like CaCl2 (both hydrogen and chlorine are in -1 oxidation state)
(5)like halogens,, hydrogen easily combines with non metal such as carbon,silicon,nitrogen etc to form covalent compounds ...
with hydrogen : CH4,SiH4,NH3
with Halogens : CCl4,SiCl4,NCl3
Answered by | 19 Mar, 2012, 12:22: PM
Application Videos
Concept Videos
CBSE 10 - Chemistry
Asked by dhumalchhaya13 | 26 May, 2022, 11:05: PM
CBSE 10 - Chemistry
Asked by kanchanbalpande82 | 12 Apr, 2022, 11:01: AM
CBSE 10 - Chemistry
Asked by kanchanbalpande82 | 12 Apr, 2022, 11:01: AM
CBSE 10 - Chemistry
Asked by waghmaresheetal78 | 27 Dec, 2021, 05:02: PM
CBSE 10 - Chemistry
Asked by ksheera36 | 03 Jun, 2021, 08:35: PM
CBSE 10 - Chemistry
Asked by vungtsaniyanthan | 16 May, 2021, 06:32: PM
CBSE 10 - Chemistry
Asked by sinhagopalakumara | 01 May, 2021, 08:16: PM
CBSE 10 - Chemistry
Asked by advssdrall | 26 Mar, 2021, 07:43: AM
CBSE 10 - Chemistry
Asked by kavithasenthil148 | 06 Feb, 2021, 07:09: PM
CBSE 10 - Chemistry
Asked by mina6poonam | 21 Jan, 2021, 04:57: PM