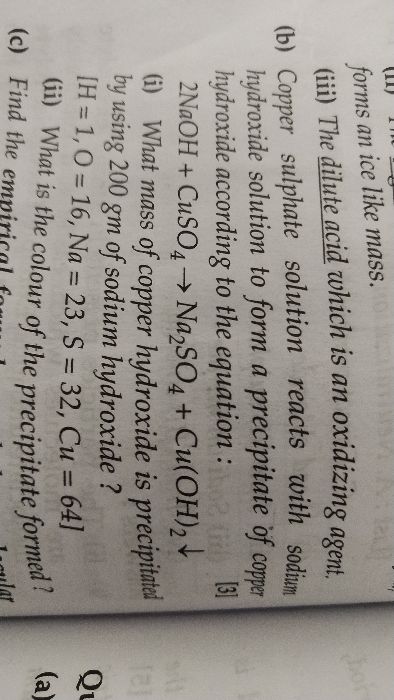ICSE Class 10 Answered
Sample of impure magnesium is reacted wih dilute sulphuric acid to give the respective salt and hydrogen. If 1g of impure sample gave 298.6 cc of hydrogen at s.t.p , calculate the % purity of the sample.
Asked by johncena9384 | 02 Oct, 2018, 10:45: PM
Impure magnesium is reacted with the dilute sulphuric acid to give magnesium sulphate and hydrogen gas.
The reaction is as,
Mg + H2SO4 → MgSO4 + H2

The purity of the sample is 32%
Answered by Varsha | 03 Oct, 2018, 10:46: AM
Application Videos
Concept Videos
ICSE 10 - Chemistry
Asked by jrvedant208 | 05 Feb, 2024, 10:37: PM
ICSE 10 - Chemistry
Asked by rashikulkarni28 | 10 Jul, 2022, 10:13: PM
ICSE 10 - Chemistry
Asked by rashikulkarni28 | 25 Jun, 2022, 10:24: PM
ICSE 10 - Chemistry
Asked by palshivom72 | 14 Jul, 2020, 07:56: PM
ICSE 10 - Chemistry
Asked by jhabijay01 | 27 May, 2020, 12:20: PM
ICSE 10 - Chemistry
Asked by aashimegh | 04 Sep, 2019, 08:53: AM
ICSE 10 - Chemistry
Asked by aashimegh | 04 Sep, 2019, 08:37: AM
ICSE 10 - Chemistry
Asked by aashimegh | 28 Aug, 2019, 05:25: PM