CBSE Class 12-science Answered
Plz answer 3.14

Asked by lovemaan5500 | 13 Aug, 2019, 17:47: PM
Given:
Molarity = 0.05 M
Resistance = 410.5 Ω
Conductance
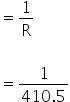
=0.0024 / ohm
Specific Conductance = Cell constant × conductance
Cell constant =

Speficific conductance = Cell constant × conductance
Cell constant is same for both the cells.
Speficific conductance K (CaCl2) =
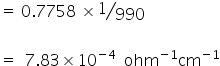
Normality of CaCl2 =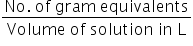
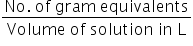
No. of gram equivalents of CaCl2 =
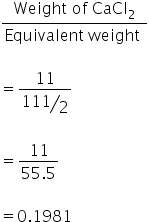
Normality of CaCl2 =
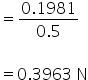
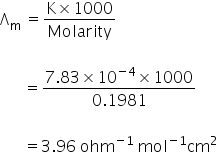
Equivalent conductance Λ=
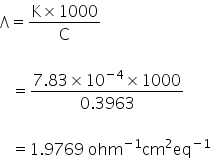
Molar conductance of CaCl2 =
Molarity of CaCl2 ,
No. of moles of CaCl2
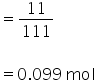
Molarity
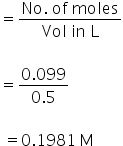
Molar conductance of CaCl2
Answered by Varsha | 14 Aug, 2019, 11:46: AM
Concept Videos
CBSE 12-science - Chemistry
Asked by skmdsajid04 | 14 Jan, 2024, 09:23: AM
CBSE 12-science - Chemistry
Asked by gauravrastogi577 | 16 Aug, 2022, 18:16: PM
CBSE 12-science - Chemistry
Asked by aarchi80 | 30 Jun, 2021, 15:37: PM
CBSE 12-science - Chemistry
Asked by chhatrashalsingh1307p | 07 Jan, 2020, 02:55: AM
CBSE 12-science - Chemistry
Asked by lovemaan5500 | 13 Aug, 2019, 17:47: PM
CBSE 12-science - Chemistry
Asked by pitambersingh260 | 23 Jun, 2018, 23:06: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 05 Jun, 2014, 10:41: AM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM






