CBSE Class 12-science Answered
ON CAROBOXLIC ACID
Asked by | 22 Feb, 2008, 09:17: PM
Carboxylic acid has COOH group and releases a H+ ion and stabilises by resonance in C=O and C-O so the C=O is not available for reaction as it is in resonance unlike aldehydes.
Answered by | 07 Dec, 2017, 07:52: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by kaziryan.05 | 29 Jun, 2021, 08:36: AM
CBSE 12-science - Chemistry
Asked by mahaynoorf | 17 Oct, 2020, 08:39: PM
CBSE 12-science - Chemistry
Asked by mufeedatvp2000 | 14 Apr, 2020, 10:58: PM
CBSE 12-science - Chemistry
Asked by mufeedatvp2000 | 14 Apr, 2020, 11:54: AM
CBSE 12-science - Chemistry
Asked by govtsecschoolnayaganv051 | 28 Aug, 2019, 07:45: PM
CBSE 12-science - Chemistry
Asked by dineshchem108 | 20 May, 2019, 11:46: PM
CBSE 12-science - Chemistry
Asked by shobhit | 21 Feb, 2019, 11:01: PM
CBSE 12-science - Chemistry
Asked by Atulcaald | 16 May, 2018, 02:43: PM
CBSE 12-science - Chemistry
Asked by Atulcaald | 16 May, 2018, 02:41: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 08 Apr, 2014, 08:33: AM




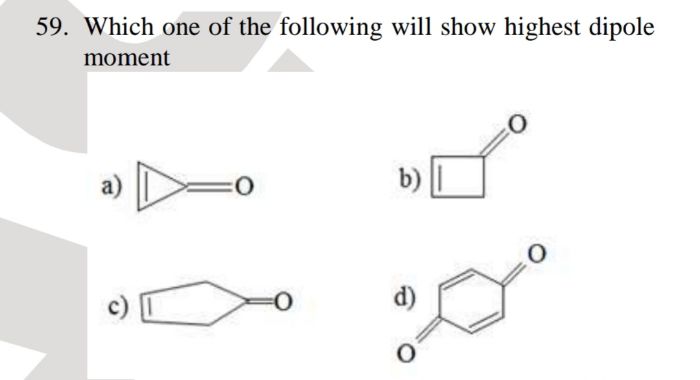
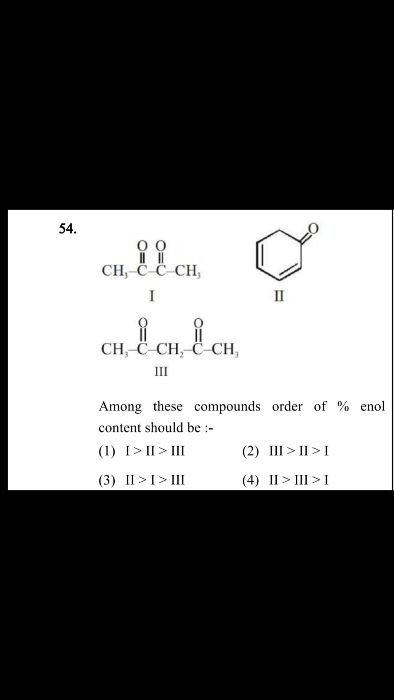
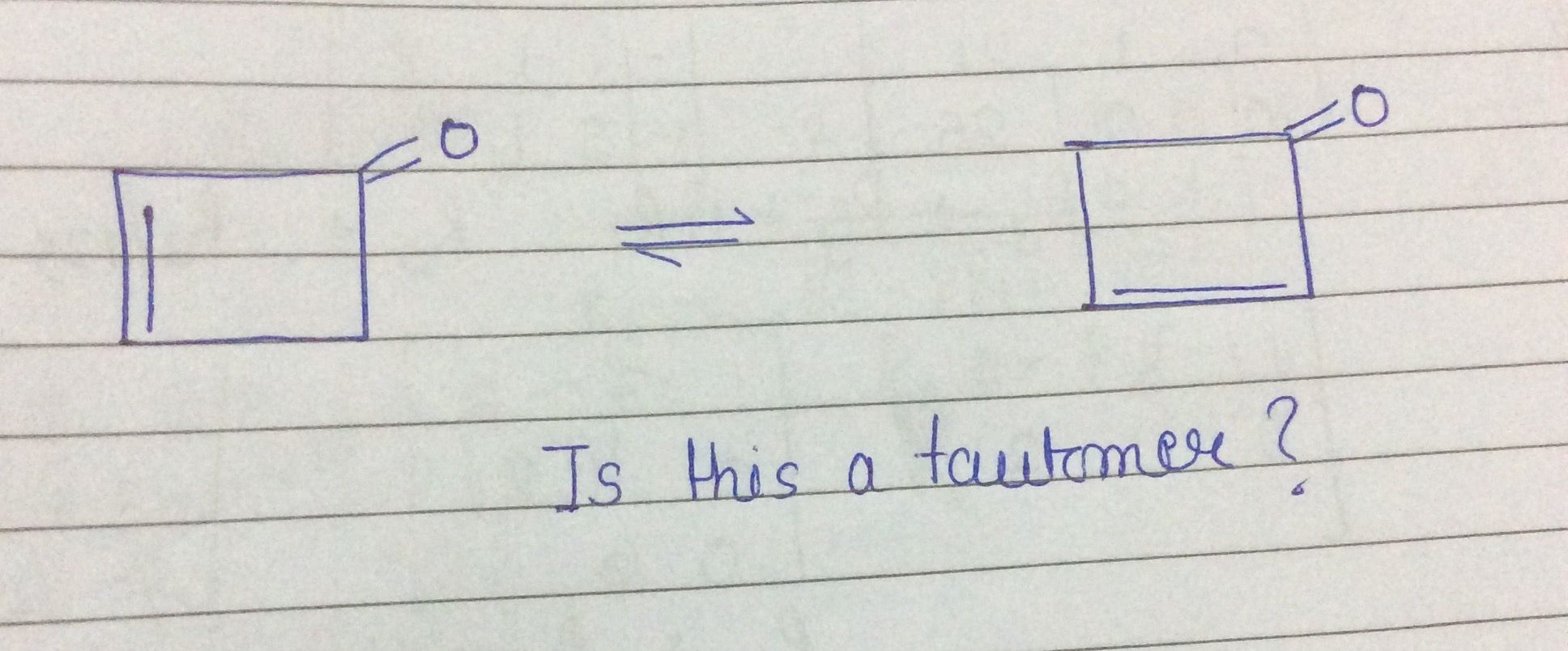
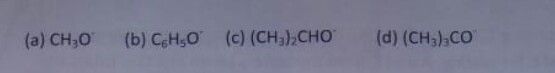
 Y reacts with Z to give
(1)
Y reacts with Z to give
(1)
 (2)
(2)
 (3)
(3)
 (4)
(4)

 Z is :-
(1)
Z is :-
(1)
 (2)
(2)
 (3)
(3)
 (4)
(4)

