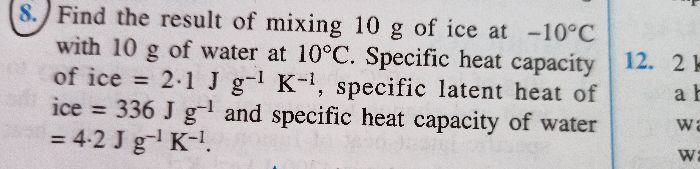ICSE Class 10 Answered
In the principle of caloriemetry we have to assume that there is no loss of heat but it is not the same practically
is the law of conservation violated in this case??
Asked by chitrachongdar07 | 08 Dec, 2020, 10:55: AM
Principle of Calorimetry:
When a hot body is mixed or kept in contact with a cold body, there is a transfer of heat from hot body to cold body such that
Total heat gained by colder body = Total heat lost by the hot body,
if there is no loss of heat to the surroundings.
When a hot body is mixed or kept in contact with a cold body, there is a transfer of heat from hot body to cold body such that
Total heat gained by colder body = Total heat lost by the hot body,
if there is no loss of heat to the surroundings.
If during practicals, if it occurs that heat lost by hot body is not equal to heat gained by cold body then it may be due to human error or instrumental error.
The principle of calorimetry is depended on law of conservation of energy and it is not violated in any practical application.
Answered by Shiwani Sawant | 08 Dec, 2020, 11:14: PM
Concept Videos
ICSE 10 - Physics
Asked by chitrachongdar07 | 08 Dec, 2020, 10:55: AM
ICSE 10 - Physics
Asked by nilesh.dhote74 | 13 Jul, 2020, 12:38: PM
ICSE 10 - Physics
Asked by nilesh.dhote74 | 10 Jun, 2020, 03:00: PM
ICSE 10 - Physics
Asked by Himadri | 16 Apr, 2020, 02:21: PM
ICSE 10 - Physics
Asked by arpitt682 | 18 Jan, 2020, 08:11: PM
ICSE 10 - Physics
Asked by om.chaudhari1673 | 23 Jan, 2019, 04:37: PM
ICSE 10 - Physics
Asked by dhruuvsinghc123 | 04 Jan, 2019, 07:41: PM
ICSE 10 - Physics
Asked by dhruvilpatel020 | 26 Jul, 2018, 11:24: AM
ICSE 10 - Physics
Asked by anish.kunwar.123 | 17 Jul, 2018, 09:11: PM